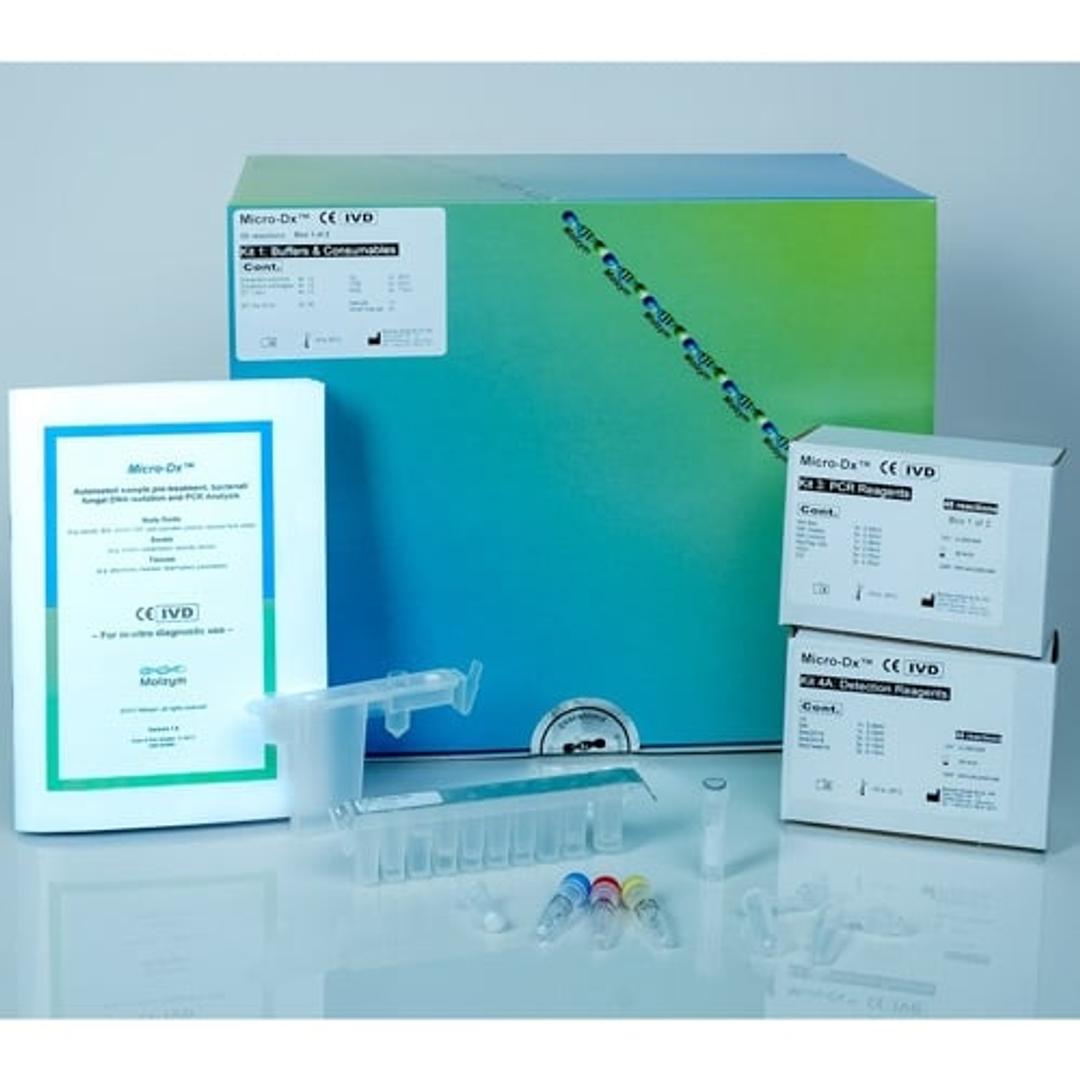
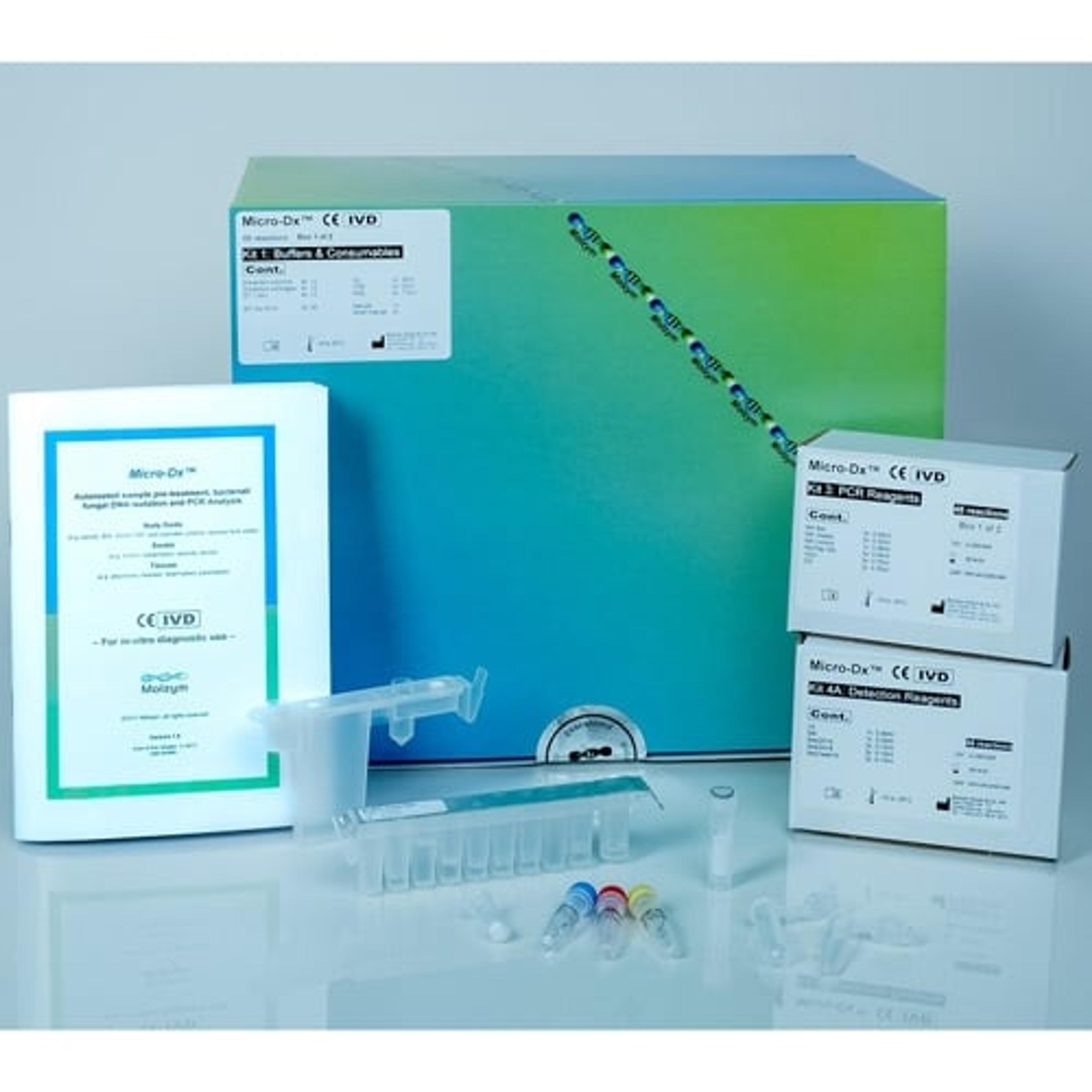
Micro-Dx™ CE IVD
Fully automated pathogen DNA isolation and broad-range PCR analysis from body fluids, swabs and tissues for clinical routine diagnostics
Micro-Dx™ CE IVD is designed for pathogen detection and identification from body fluids, swabs and tissues independent from culture. The fully automated sample preparation includes host DNA depletion for a more sensitive detection of bacteria and fungi in clinical samples. The broad-range PCR assays deliver first results after approximately four hours. Subsequent Sanger sequencing can identify the pathogens down to species level.
Benefits:
- Reduced hands-on time
- Variety of specimens: body fluids, swabs, tissues
- Flexible: 1- 12 samples
- Increased sensitivity by depletion of host DNA
- DNA-free reagents and consumables
- Broad-range PCR assays for the detection of bacterial and fungal DNA
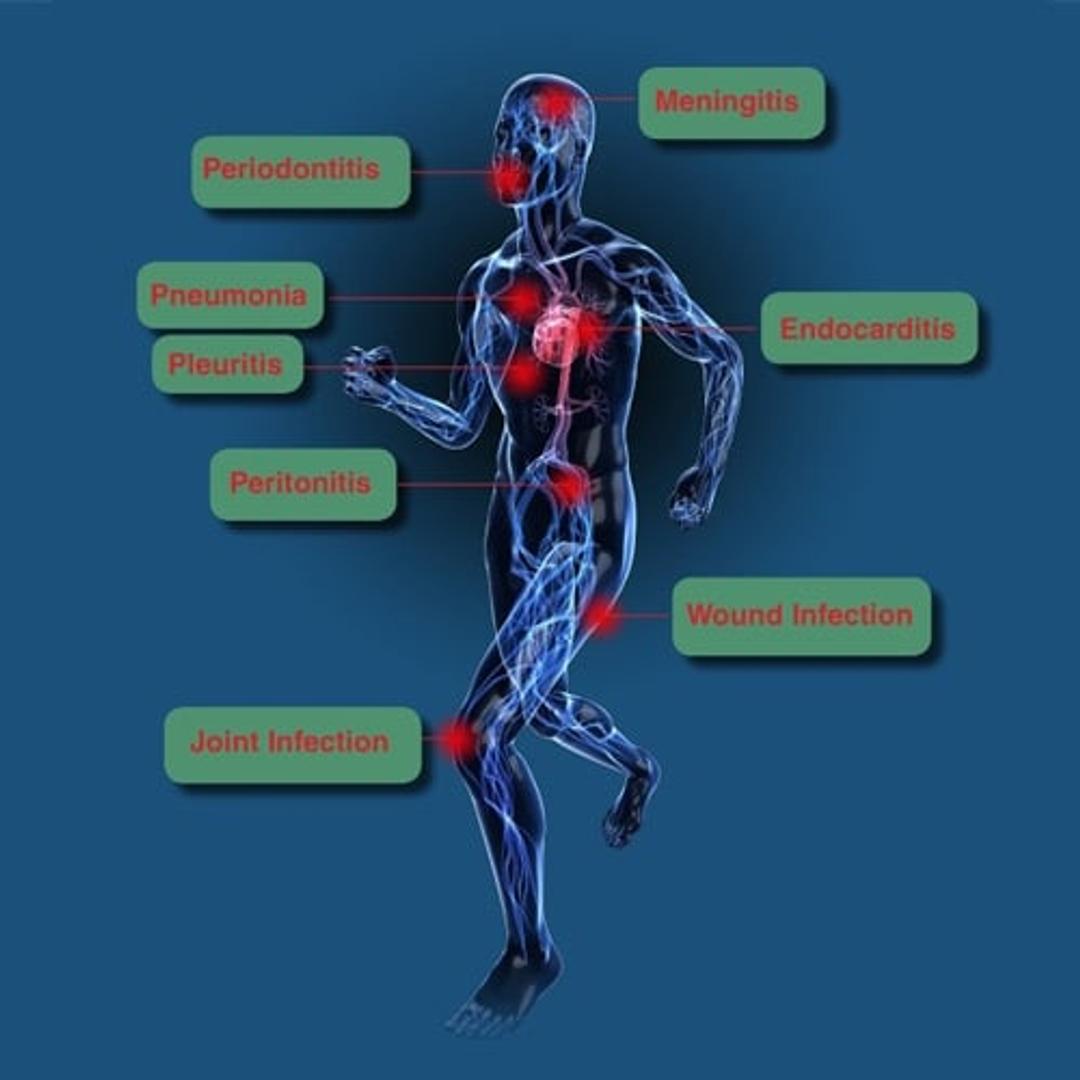
In most samples, host DNA is in vast excess to target microbial DNA which often reduces the sensitivity of molecular analysis significantly. Therefore, a reduction of host DNA leads to an increased sensitivity in the detection of microbial DNA. The DNA-free reagents provided with the kit and the automated DNA isolation commit to the contamination-free processing of the samples. 16S and 18S rDNA-based broad-range PCR assays are used for the sensitive detection of bacterial and fungal DNA, respectively. More than 1.300 different pathogens have already been detected in clinical studies and evaluations. Micro-Dx™ can identify pathogens causing e.g. sepsis, bacterial meningitis, pneumonia, joint and wound infections.