News & Articles
Don Whitley Scientific launches smallest ever anaerobic workstation
Anaerobic chamber is designed for processing, incubation and examination of samples without exposure to atmospheric oxygen
Abbott receives FDA approval for new heart rhythm devices featuring Bluetooth connectivity and continuous remote monitoring
Gallant ICD and CRT-D devices feature a patient-preferred design without compromising on battery longevity and compatibility with magnetic resonance imaging (MRI)
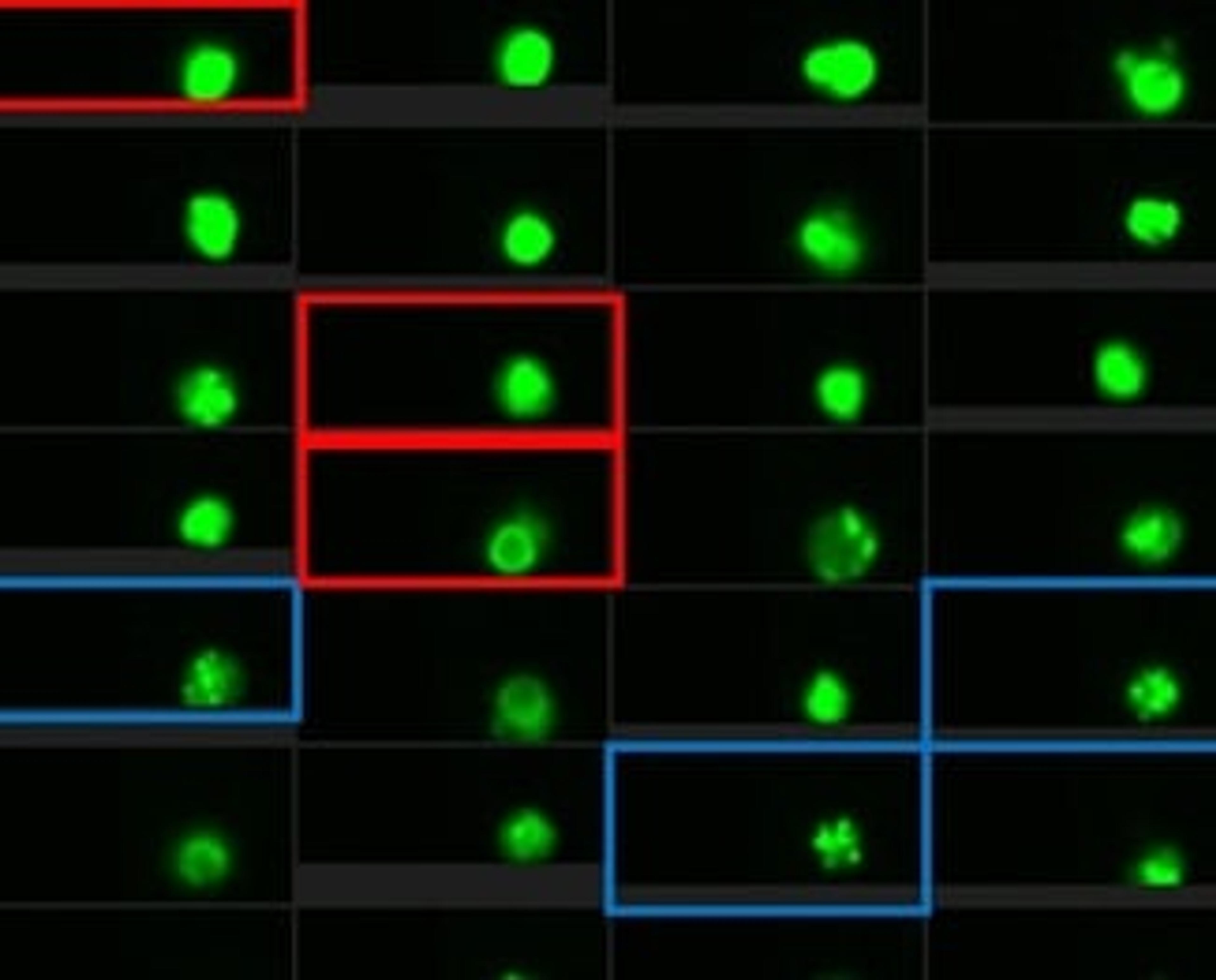
Using DNA repair and machine learning to improve cancer risk prediction
Scientists are harnessing machine learning to make accurate measurements of breast cancer risk from a patient’s blood cells
Best practices for rapid detection of acute cardiovascular disease
Prof. Ishii explains how high-sensitivity troponin I assays and a 1-hour algorithm can be best used for early diagnosis and treatment of myocardial infarction
Rapid in-solution equilibrium approach to determine binding affinity of biotherapeutic drug candidates
Join us on Monday, July 20, to discover the benefits of in-solution equilibrium affinity experiments using Gyrolab systems
Win a VACUSAFE Aspiration System from INTEGRA
Synergy H1’s continuously variable bandwidth monochromators optimize assay performance
These monochromators aim to achieve greater levels of assay sensitivity and specificity compared to fixed bandwidth systems
CRAIC Technologies announces the addition of UV-visible-NIR transmission and reflectance polarization spectroscopy
This unique feature is offered as a package that allows the user to measure polarization spectra in either transmission or reflectance modes
Join the SelectScience webinar series on COVID-19 and Infectious Disease
Starts Monday, July 6. Register for free today to stay informed on the latest techniques and technologies for COVID-19 and infectious disease research
Navigating through the pandemic: The latest on COVID-19 testing
Join us on Thursday, July 16, to learn about the landscape for COVID-19 testing, the latest methods and tests, and the current state of regulations
Perfecting automated solid phase extraction for PFAS and other perfluorinated compounds in drinking water
Learn how one U.S. analytical lab has struck a partnership with its technology provider to become an industry leader in drinking water analysis
Secondary ion mass spectroscopy: Sensitive compositional analysis at the micro- and nanoscale
Watch this on-demand workshop to gain insight into SIMS and discover the new ZEISS SIMS portfolio
First exposed planetary core discovered
Researchers led by the University of Warwick have discovered the first exposed core of an exoplanet, which provides an unprecedented glimpse inside the interior of a planet
Abbott, Tandem Diabetes Care advance development of technologies for insulin delivery systems
Companies partner to integrate Abbott's FreeStyle Libre continuous glucose monitoring technology with Tandem's insulin delivery products to provide options to simplify and tailor diabetes management
Corning launches X-SERIES cell processing platform to support cell and gene therapy applications
The platform aims to deliver rapid and automated processing of human blood and blood products to obtain purified populations of immune cells
Sherlock Biosciences, binx health partner to develop first CRISPR-based point-of-care test for COVID-19
binx health’s proven molecular io platform combined with SHERLOCK CRISPR technology will enable rapid, accurate testing for SARS-CoV-2 in retail and near-patient settings
Extensive range of high integrity microplate heat sealing films
Porvair Sciences has expanded its range of high integrity heat sealing films