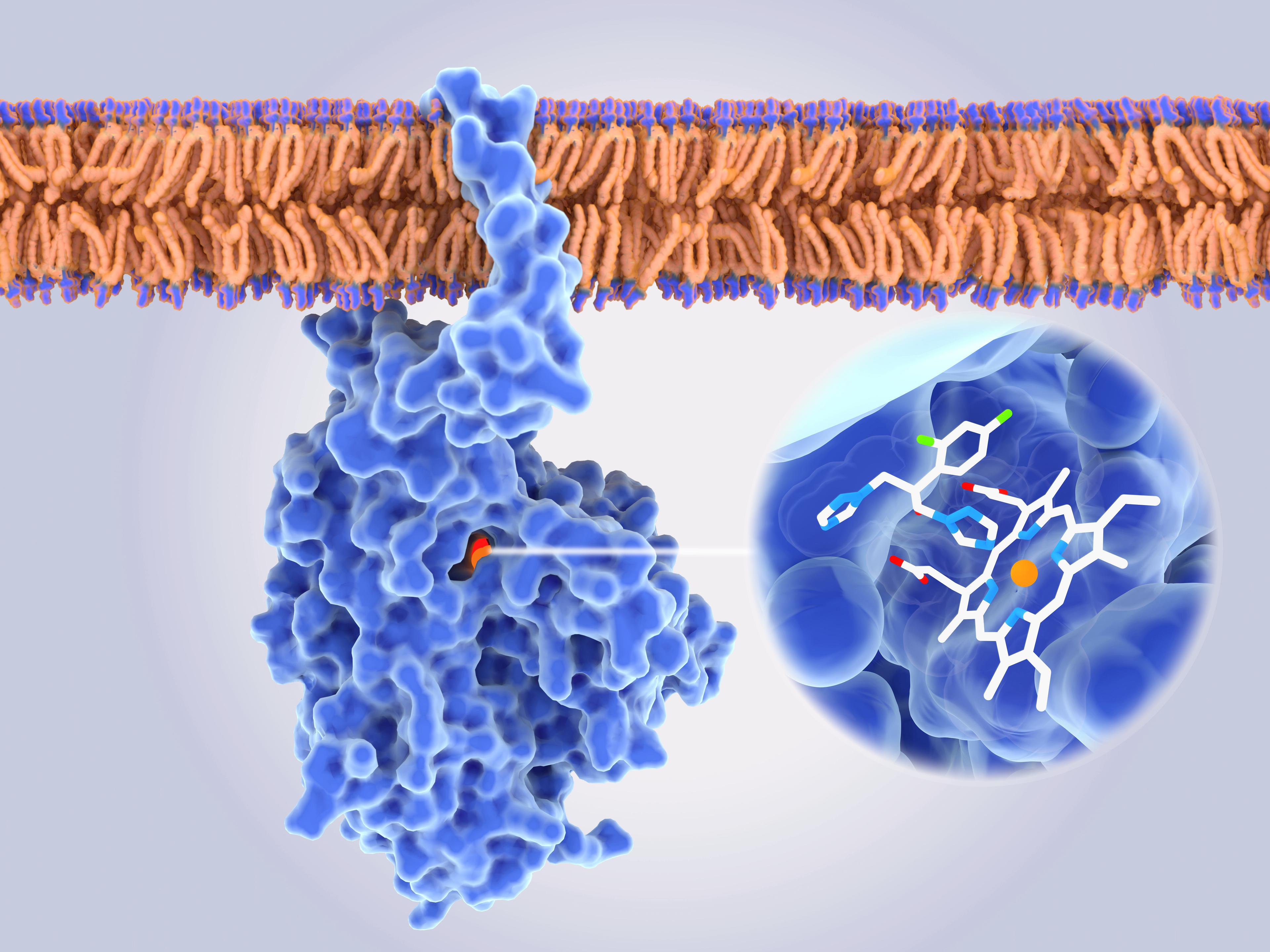
Advances in bioanalytical method development
Bioanalytical method development is undergoing a transformation, driven by the need for greater sensitivity, specificity, and speed in the analysis of complex biological matrices. As the demand for robust data grows across drug development, the pressure to deliver accurate, reproducible, and regulatory-compliant methods has never been higher.
Innovations in analytical instrumentation, automation, and data processing, from high-resolution mass spectrometry and microfluidics to AI-assisted workflows and digital validation, are redefining how bioanalytical methods are designed, optimized, and validated. These advances are bridging the gap between early-stage research and regulated environments, ensuring data integrity and accelerating time to insight.
This dedicated feature explores the technologies, strategies, and digital tools shaping the future of bioanalytical method development. Discover expert insights, emerging trends, and curated resources to support innovation and decision-making across the bioanalysis landscape.
Explore why testing for pre-existing AAV antibodies is essential for successful gene therapy outcomes, and how Gyros Protein Technologies is helping to address this need.
Read article