Resources
25
Product Brochures
Introducing the Omnia Discovery
Application Notes
Food microbiology testing methods for Listeria species
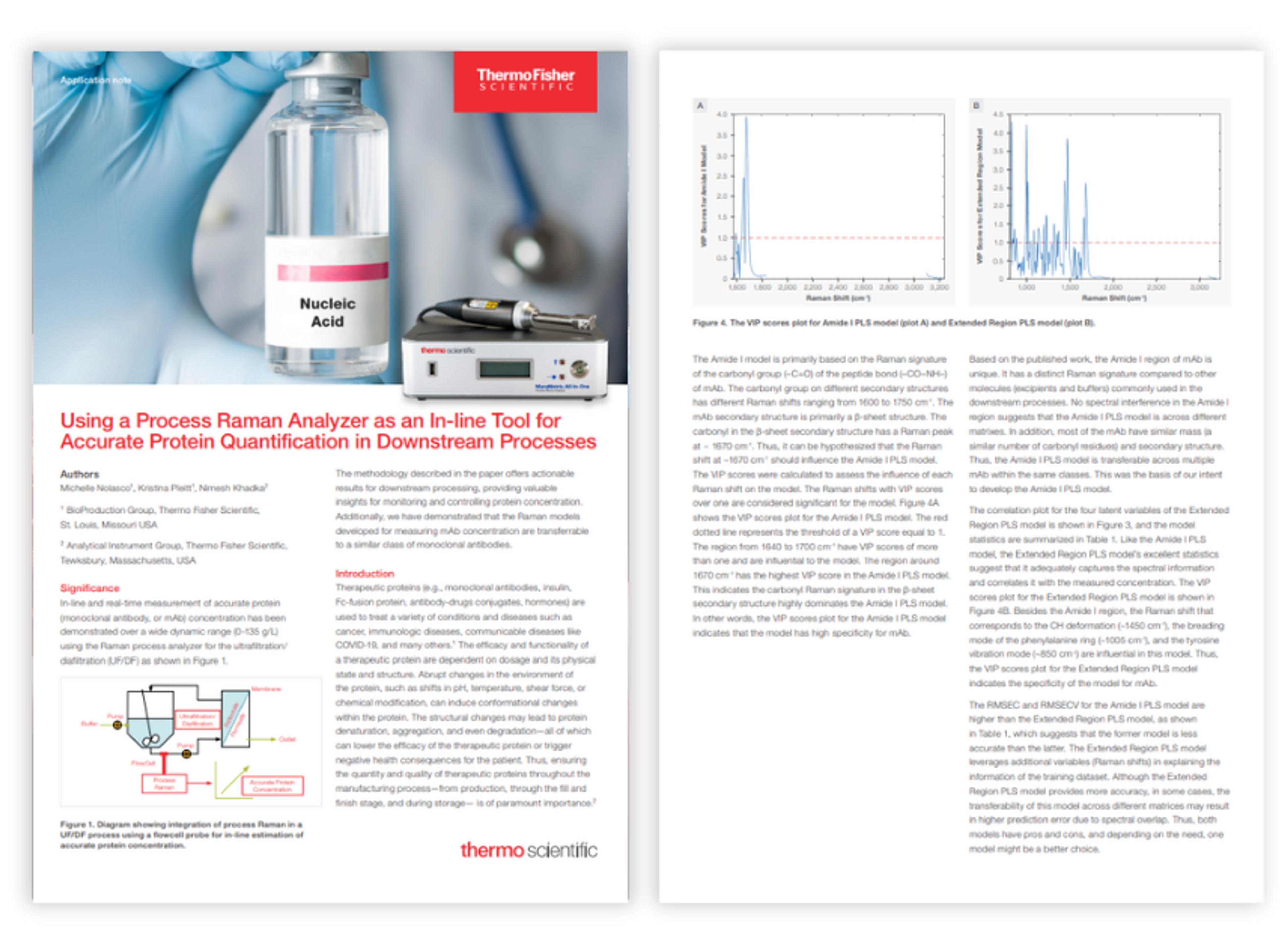
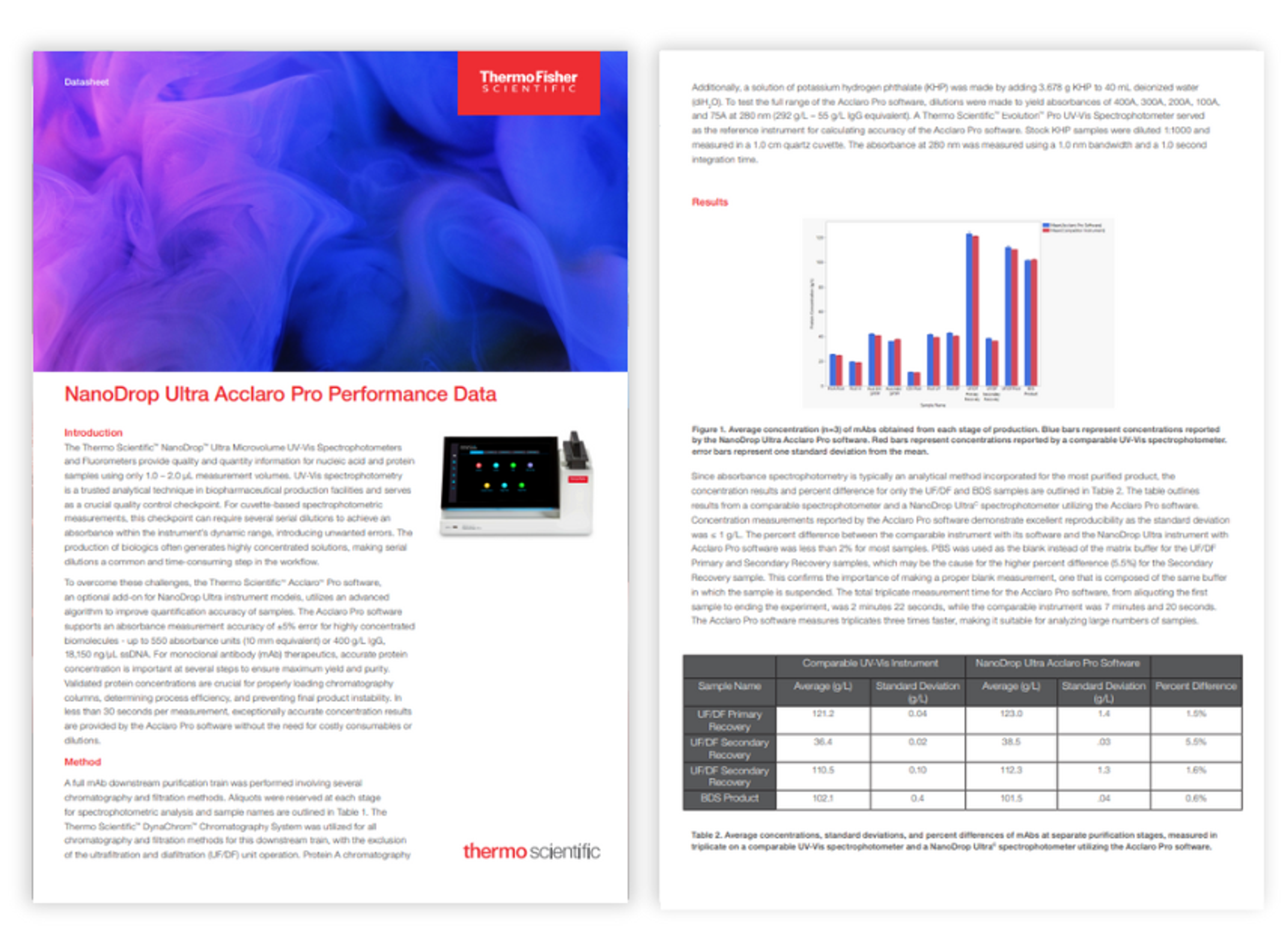
Application Notes
NanoDrop Ultra Acclaro Pro performance data
Application Notes
Rapid electrochemical assessment of paint
Product Brochures
Pipetman M96: Tips and tricks
Product Brochures
LICORbio Atlas Imaging System
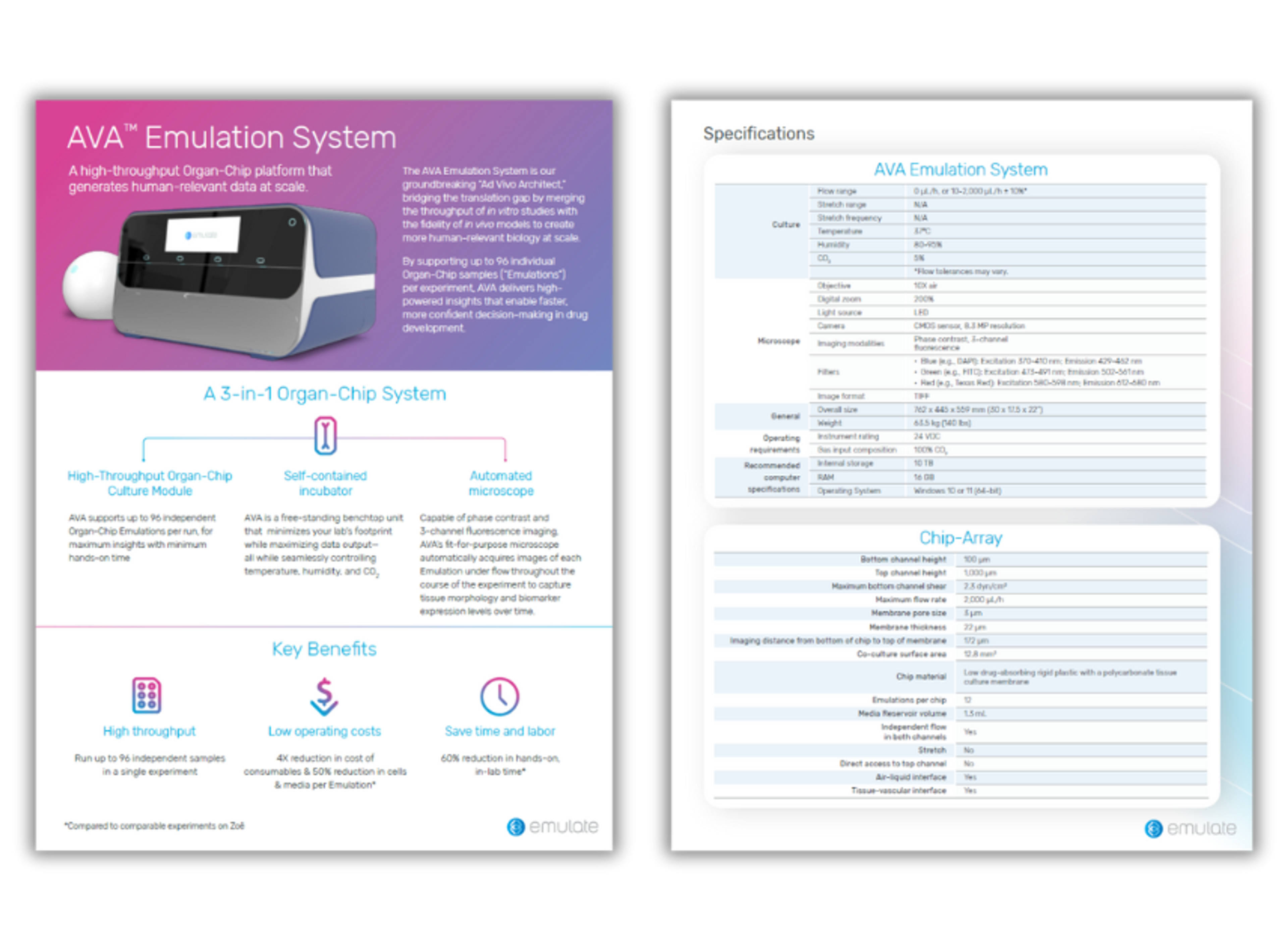
Product Brochures
AVA Emulation System
Application Notes
Tailored strategies for lab automation
Application Notes