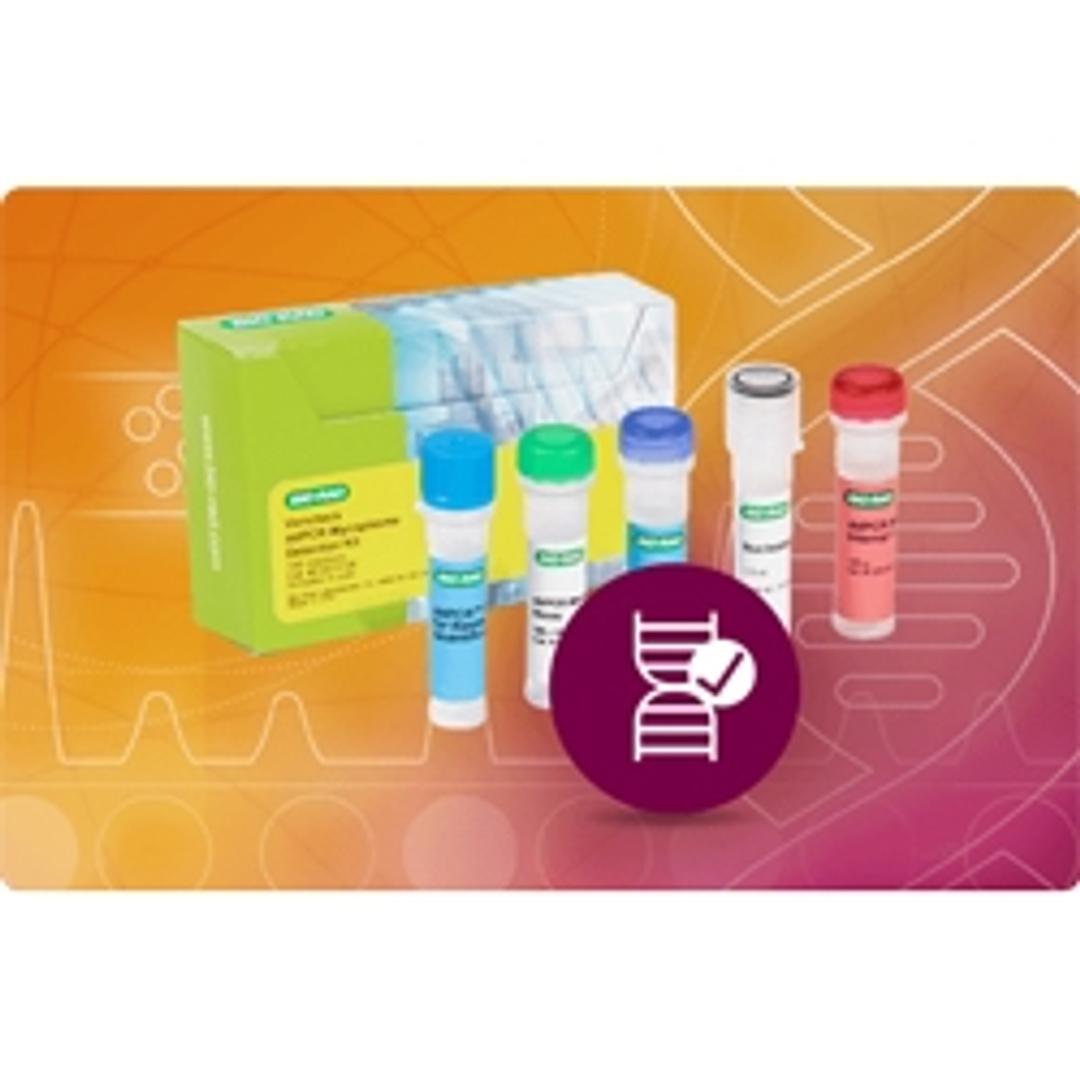
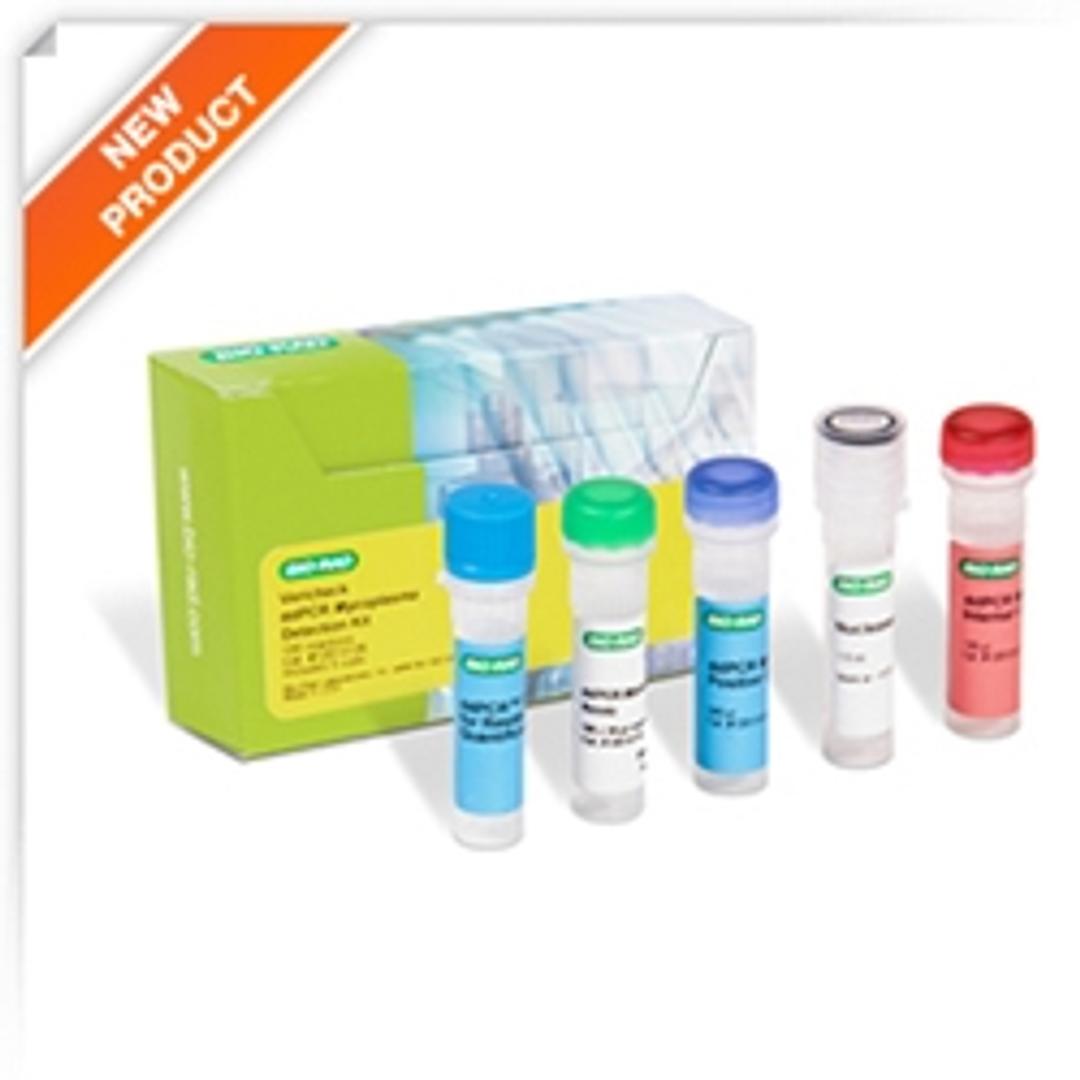
Vericheck ddPCR Mycoplasma Detection Kit
The probe-based Vericheck ddPCR Mycoplasma detection Kit brings confidence in testing for mycoplasma contamination. The specificity and reproducibility of this kit allows for a precise measurement of 112 mycoplasma species using droplet digital PCR.
Mycoplasma is a commonly found contaminant in biopharmaceutical development processes that are typically not detectable by standard microscopic methods due their small size.The European, U.S., and Japanese pharmacopoeias define the levels of mycoplasma that should be present in any given product. Bio-Rad’s Vericheck ddPCR Mycoplasma Detection Kit is the first droplet digital PCR based mycoplasma testing solution. The sensitive, probe-based kit with high specificity has been designed and validated to meet European, U.S., and Japanese pharmacopoeia requirements and can detect up to 112 mycoplasma species. The QX Manager software includes tools to help with FDA 21 CFR Part 11 compliance along with a new positive control-based auto-thresholding feature that has been added for turnkey, easy data analysis.
Features and benefits
- Probe-based test
- Multiplex probe-based assay kit designed and Validated to meet requirements from European Pharmacopoeia (EP), U.S Pharmacopoeia (USP) and Japanese Pharmacopoeia (JP)
- High sensitivity and specificity
- LoD studies determine the lowest detectable concentration of mycoplasma (<6 Genome Copies/Well OR 1 CFU/ml)
- Low cross-reactivity with the related species, providing low false-positive results
- Auto thresholding with Regulatory Compliant Software
- Auto gate your results based on a positive control, removing manual intervention/manual data interpretation and streamlining data analysis.
- Audit trails with tracked protocol changes for regulatory requirements
Applications and uses
- Detecting presence/absence of mycoplasma in drug products
- Quantitative data analysis providing confirmation on the quantity of mycoplasma