Products & ReviewLife Sciences
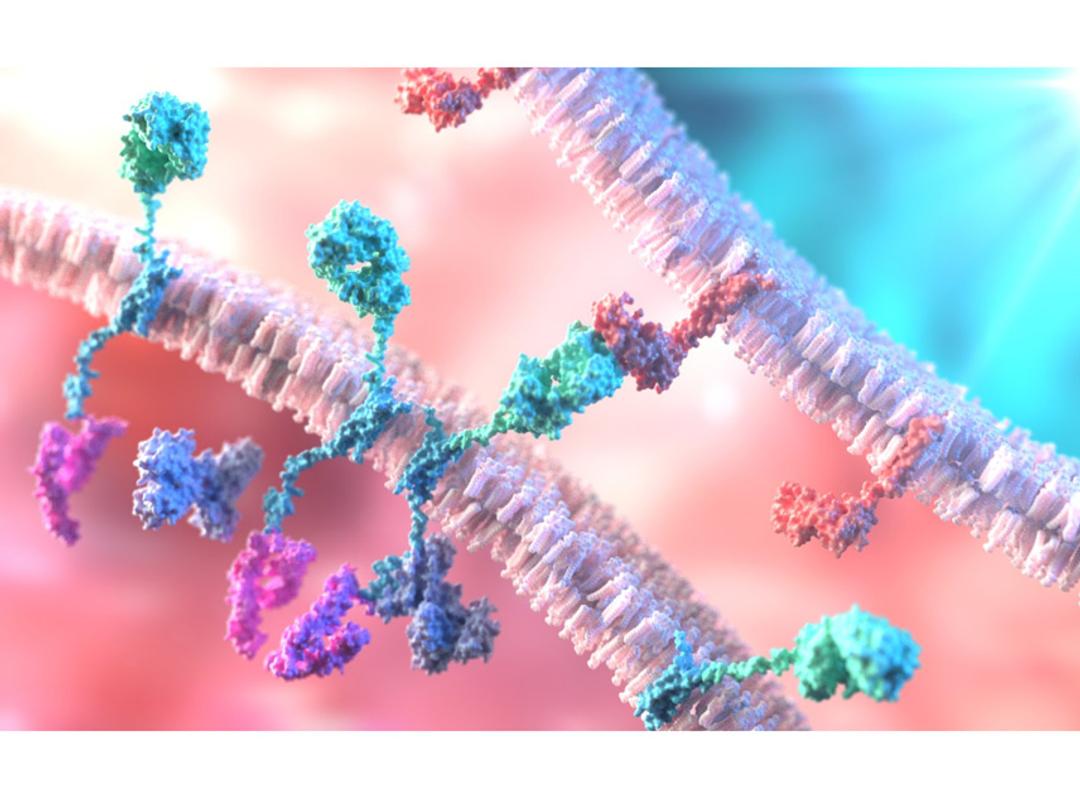
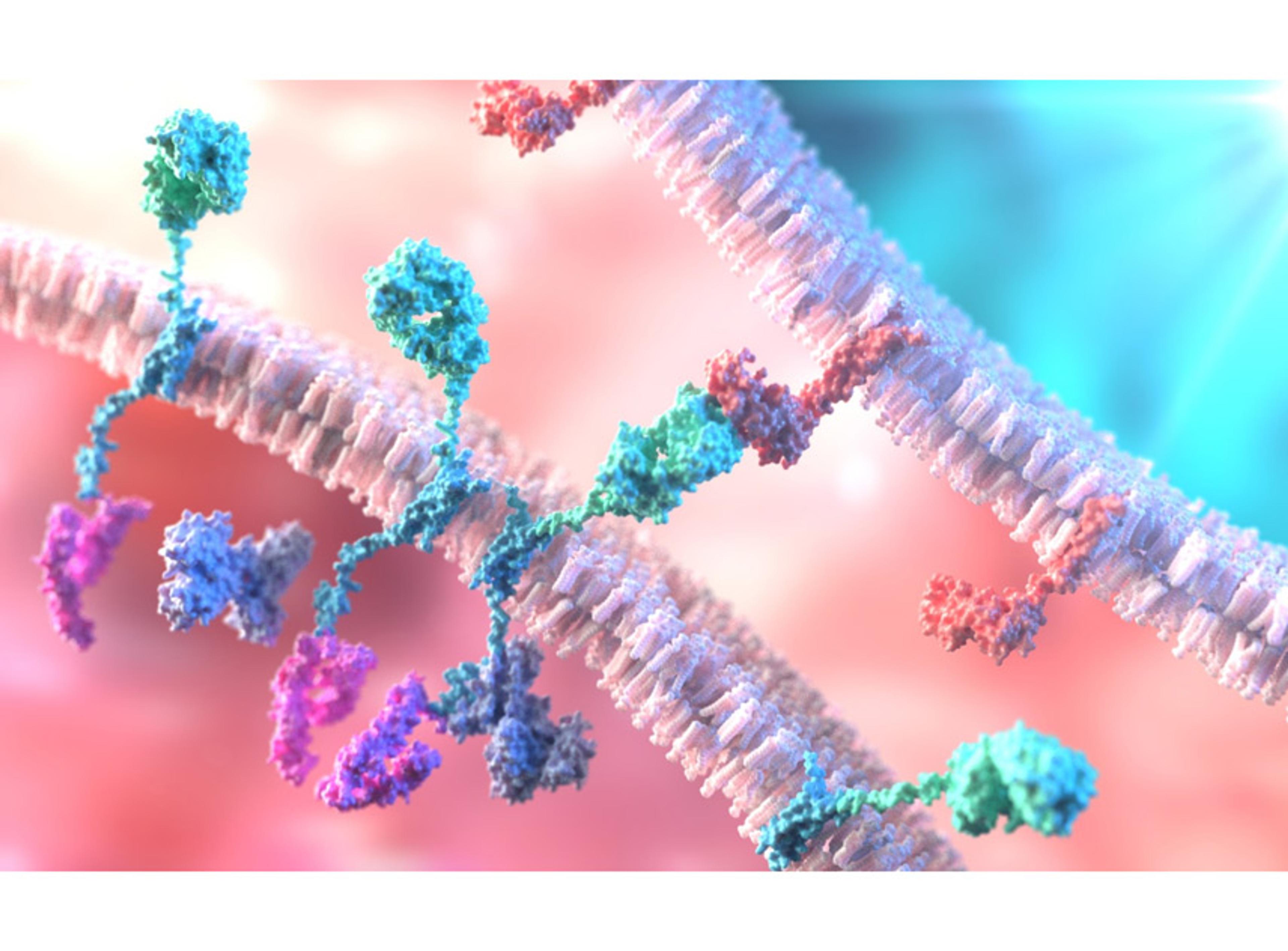
Targets of CAR-T Cell Therapy
A portfolio of recombinant immunoglobulins and cell surface proteins serving as critical targets for the development and functional validation of Chimeric Antigen Receptor T-cell (CAR-T) therapies.
CAR-T therapy involves engineering a patient's T-cells to express a receptor that recognizes a specific antigen on tumor cells. These target proteins, such as CD19 and BCMA, are used as reagents to test the binding and efficacy of CAR constructs, perform potency assays, and ensure the specificity of the therapeutic product.
Key Features & Benefits:
- Includes Key Tumor Antigens (e.g., CD19, BCMA, CD22)
- Multiple Formats (Monomeric, Dimeric, Fc-fusion) for CAR Validation
- High Purity and Activity for Reliable Bioassays
- Supports Specificity and Cross-Reactivity Testing
Relevant Applications:
- CAR-T Cell Binding and Functional Validation
- Potency Assay Development and Lot Release Testing
- Specificity and Off-Target Risk Assessment
- CAR Engineering and Affinity Maturation