LAMP-based diagnostics: Rapid and truly portable molecular testing at the point-of-need
Discover how a multi-disciplinary team located at Imperial College London created a novel portable diagnostic platform and delivered a rapid and accurate 5-in-1 respiratory pathogen detection test panel
12 Jan 2023

Considered by many to be the ‘gold standard’ in molecular diagnostics, quantitative polymerase chain reaction (qPCR) has revolutionized how we diagnose and investigate infectious diseases. This revolution however, is one most realized by those who can afford the necessary technology, and by those with sufficient training and access to a well-equipped laboratory. Nations with fewer resources, or scientists working in the field or in restricted settings, are therefore put at a disadvantage.
Health technology spin-out, ProtonDx, was established in 2020, bringing together some of the greatest minds and multi-disciplinary research developed at Imperial College London, with the aim of delivering an alternative, portable, and low-cost diagnostics solution that leaned on the inherent advantages of highly sensitive loop-mediated isothermal amplification (LAMP) technology.
Speaking with ProtonDx co‑founder and CSO, Dr. Jesus Rodriguez Manzano, and Chairman and President, Bob Enck, SelectScience® explores this exciting technology in greater detail and the positive impact it is already having at the point-of-need with the new portable testing platform, Dragonfly.
The intrinsic benefits of LAMP
First developed in 2000, LAMP is a fast method of amplifying nucleic acids with high sensitivity and specificity, without the need for the expensive reagents or instruments used in PCR. “LAMP has two very big advantages that truly enable detection at the point-of-care or point-of-need. One, it can be as sensitive as traditional PCR but with the advantage of speed. We have been able to get a positive result in as fast as five minutes. Though, we run our tests for up to 30 minutes to be sure that we capture low concentrations in a sample. Our team is very experienced with developing LAMP assays, so we are able to push detection to the limit,” explains Dr. Rodriguez Manzano. “Two, it's isothermal, so you remove the need for thermocycling. This is one of the big limitations of molecular testing at the point-of-care, because it's very difficult to design a small and affordable thermocycler with the required performance.”
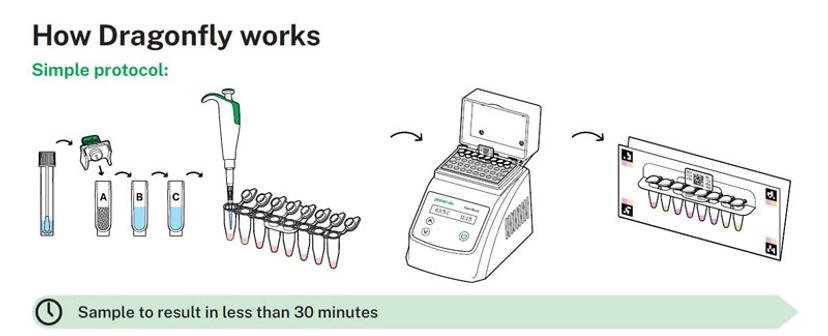
Without the need for an expensive, heavy thermocycler, Dragonfly benefits from a very light and small heating element, which runs at a consistent temperature throughout the 25-minute reaction time. Dr. Rodriguez Manzano notes this as a particular strength of the system when looking at low-resource and in-the-field settings. He goes on to describe how the innovative approach to RNA and DNA sample preparation yields similar advantages, “Part of the reason our sensitivity and specificity is so good is because we extract a very pure sample. The whole kit is sample to result, and that's enabled thanks to our proprietary SmartLid™ component where we are using paramagnetic beads to enable the sample preparation process.”
Rapid identification of multiple respiratory pathogens
As COVID-19 vaccine programs began to see success and social restrictions were lifted in many areas of the world, a resurgence in other respiratory pathogens including influenza A, influenza B, respiratory syncytial virus (RSV), and human rhinovirus was observed. ProtonDx was quick to respond, “We listened to the marketplace and understood that there was a need for a rapid, accurate, multiplexed, molecular-level test that’s portable and could identify the presence of these four different pathogens as well as SARS-CoV-2”, Enck explains. “As a team, we have the knowledge and capability to turn around a new pathogen detection panel in the order of weeks, and so we can be flexible as needs shift over time.”

Enck describes how the speed and high sensitivity of the CE‑IVD marked Dragonfly Respiratory Panel they created, paired with the compact form factor of the platform, means it can be deployed in a raft of environments, “Thinking of care home patients and at-home healthcare, or in the hospital or general practice where a patient presents with respiratory symptoms, in less than 30 minutes, sample to result, you can do a swab and identify if they have one of those five viral targets,” he deliberates.
This utility does not stop there however, “Most recently, we’ve seen the platform being used at elite sporting events such as the 2022 Commonwealth Games to enable timely treatment decision making for athletes, and therefore minimizing unnecessary antibiotic use. It provided peace of mind to athletes and coaches in the run‑up to, and during, the Games.”
Reaching ‘gold standard’ performance through collaboration
The development of ProtonDx’s technologies and products has been supported by both internal collaboration at Imperial College London and partnerships with experienced industry suppliers. “Because we are delivering a solution which is comparable to the gold standard, we need to have access to the best reagents. Of course, we have developed a series of in-house solutions to support our technology so far, but it would have taken significantly longer to reach the point where we are at now if we did this for every component. So for us, it was beneficial to partner with external suppliers, for example reagent suppliers such as LGC Biosearch Technologies, during our proof of concept stages,” states Dr. Rodriguez Manzano.
Enck adds, “Companies like LGC also have relationships with other firms in the industry. So, to be able to reach out to them and say, ‘Hey, we're thinking about this new test or new market. Who do you know and recommend as partners or collaborators?’ on either the commercial or manufacturing front has been helpful, for particular situations.”
An immediate and lasting impact
On top of being a co-founder and CSO of ProtonDx, Dr. Rodriguez Manzano is also a leading academic researcher with a profound interest in tackling antimicrobial resistance and improving antibiotic stewardship.1-3 With the Dragonfly Platform, he and the ProtonDx team are addressing the need for improved diagnostics as one of the 10 different actions under the UK government’s 5-year action plan for antimicrobial resistance, 2019 to 2024. “We need to have diagnostics that can be used on the front line, in GP’s clinics, for example, as well as in low-income or low-resource settings, in order to provide better treatment for patients. An accurate diagnosis can guide treatment decisions and in turn, improve infection prevention and control measures,” he explains.
Looking to the near future, the ProtonDx team are exploring other areas where the platform could bring simplicity, confidence, and certainty to patient testing. “We have also developed a companion app to be used on a tablet or mobile phone, which can automatically generate a report, store it in the cloud, and provide important connectivity aspects that could be required in the future for population surveillance/epidemiological studies and the screening of infectious diseases,” states Dr. Rodriguez Manzano.
References
1. National Institute for Health and Care Research, 2022. Antimicrobial resistance research: learning lessons from the COVID-19 pandemic. Academy of Medical Sciences.
2. Charani E, McKee M, Ahmad R, et al. 2021. Optimising antimicrobial use in humans - review of current evidence and an interdisciplinary consensus on key priorities for research. Lancet Reg Health Eur, 29(7).
3. Ming D, Rawson T, Sangkaew S, Rodriguez-Manzano J, Georgio P, and Holmes A. 2019. Connectivity of rapid-testing diagnostics and surveillance of infectious diseases. Bull World Health Organ, 97(3).