Turning ‘undruggable’ proteins into therapeutic targets
Explore the future of membrane protein therapeutics with innovative antibody platforms
18 Dec 2025Guest article by Creative Biolabs.
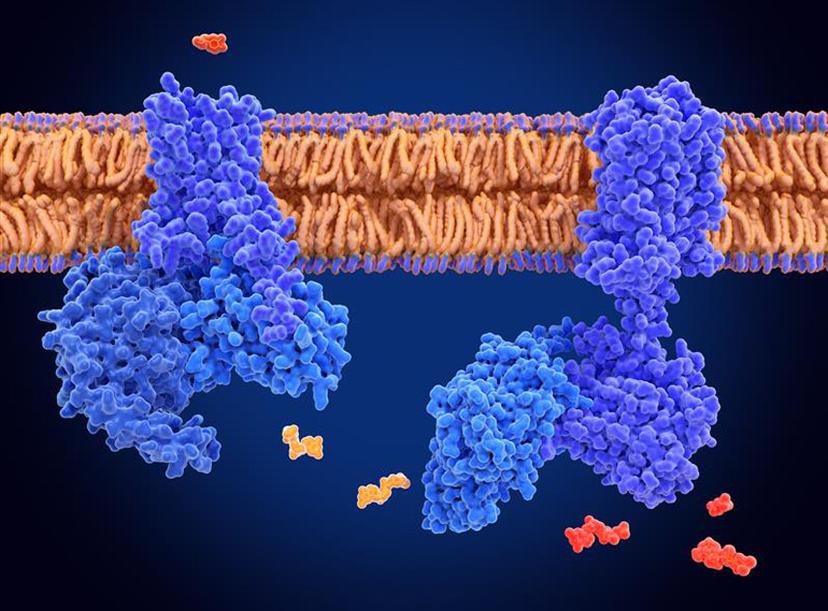
G-protein coupled receptors
In the landscape of modern therapeutics, membrane proteins represent the industry's most valuable and, simultaneously, most frustrating class of targets. This extensive family—encompassing G-protein-coupled receptors (GPCRs), ion channels, transporters, and complex receptors—acts as the cell's gatekeepers, controlling the flow of information and materials. It's no exaggeration to say they are the "holy grail" of drug discovery; it is estimated that over 60% of all approved drugs exert their effects by modulating these proteins.
Yet, for the biologics sector, this grail has been largely out of reach. While small molecules have successfully targeted these proteins for decades, the development of highly specific, functional monoclonal antibodies (mAbs) has been a profound challenge. The core issue is one of profound biophysical difficulty. We're not just targeting proteins, but their conformations.
The conformational challenge: The integrity crisis
Membrane proteins are fundamentally different from their soluble counterparts. They are born and survive in the oleophobic, hydrophobic environment of the cellular lipid bilayer. The moment you extract them using traditional detergents for use as an immunogen or screening target, you are pulling them from their native home. More often than not, their delicate transmembrane domains collapse, and the protein denatures.
This leads to the primary failure of early discovery campaigns: antibodies are generated, but they bind to linear, non-functional epitopes on a "dead" protein. These binders invariably fail to recognize the native, correctly folded protein on the surface of a live cell. This is a critical and costly dead end.
The solution: An integrated, multi-platform strategy
Success in this field is no longer about finding a single "magic bullet." Instead, it requires a sophisticated end-to-end strategy that integrates multiple advanced platforms. Creative Biolabs offers one approach, incorporating three pillars to preserve crucial native conformations.
Pillar 1: The 'Antigen is King' philosophy
Your antibody is only as good as the antigen you use to generate it. The industry's greatest leaps forward have been in antigen preparation.
- Virus-like particles (VLPs): This is a powerful immunization strategy. Creative Biolabs co-expresses the target membrane protein with a viral structural protein (like Gag), which then self-assembles and buds from the host cell. The result is a non-infectious "particle" that densely studs its surface with the target protein, all in its native membrane environment. This high-density, high-integrity presentation is exceptionally immunogenic.
- Nanodiscs: For in vitro screening (like Phage Display) or biophysical analysis (like SPR/BLI), nanodiscs are revolutionary. Creative Biolabs uses a "belt" of membrane scaffold protein (MSP) to encircle a small patch of lipid bilayer, "capturing" a single, stable membrane protein molecule. This creates a soluble, homogenous, and stable target that fully exposes its extracellular domains.
- Whole-cell immunization: Why extract the protein at all? Creative Biolabs can immunize transgenic mice directly with host cells that are engineered to over-express the target protein. This guarantees the immune system only sees the protein in its 100% native state, surrounded by its natural lipid and protein partners.
Pillar 2: Deploying the right discovery engine
Once you have a high-integrity antigen, you must choose the right discovery engine to find your binder.
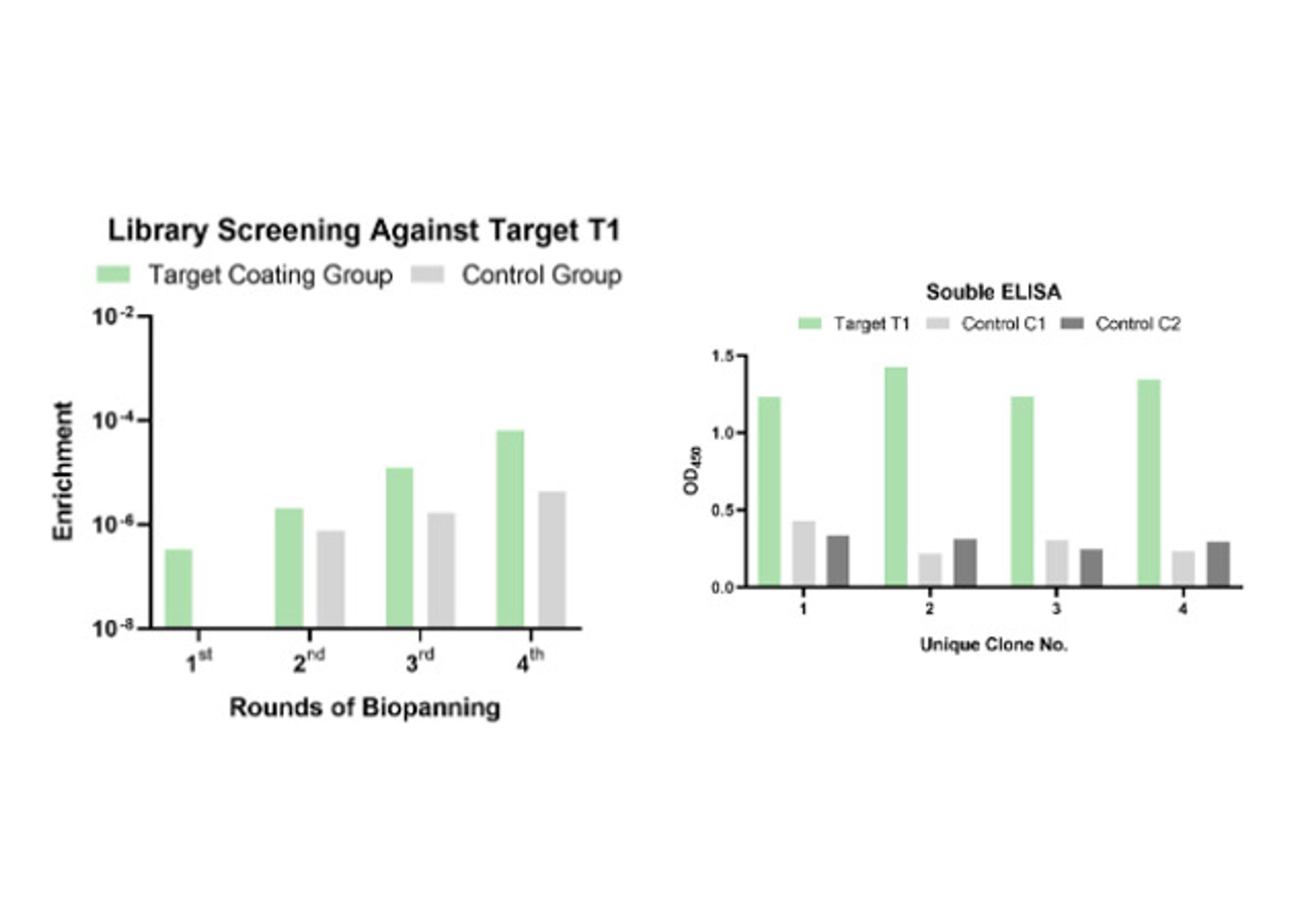
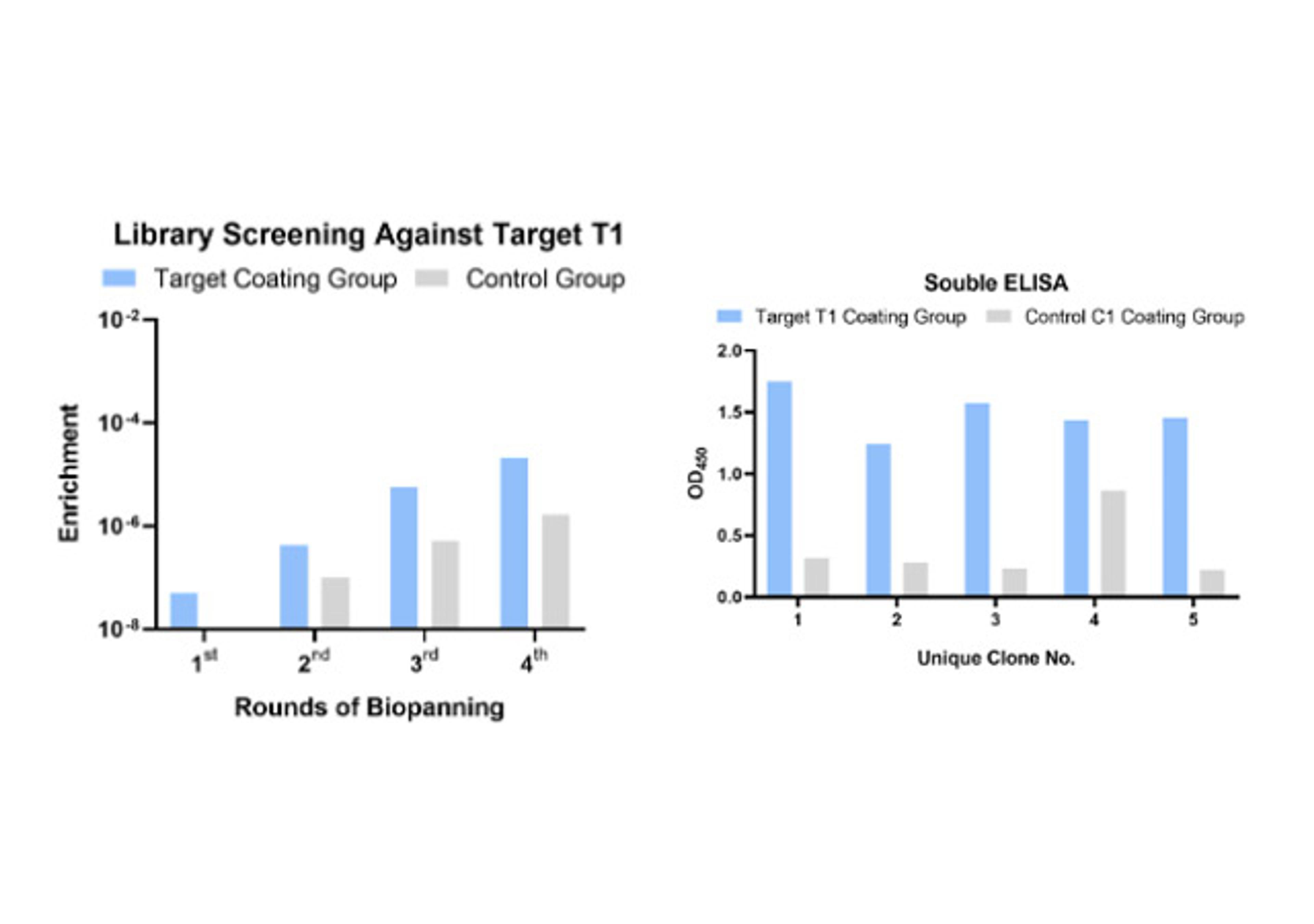
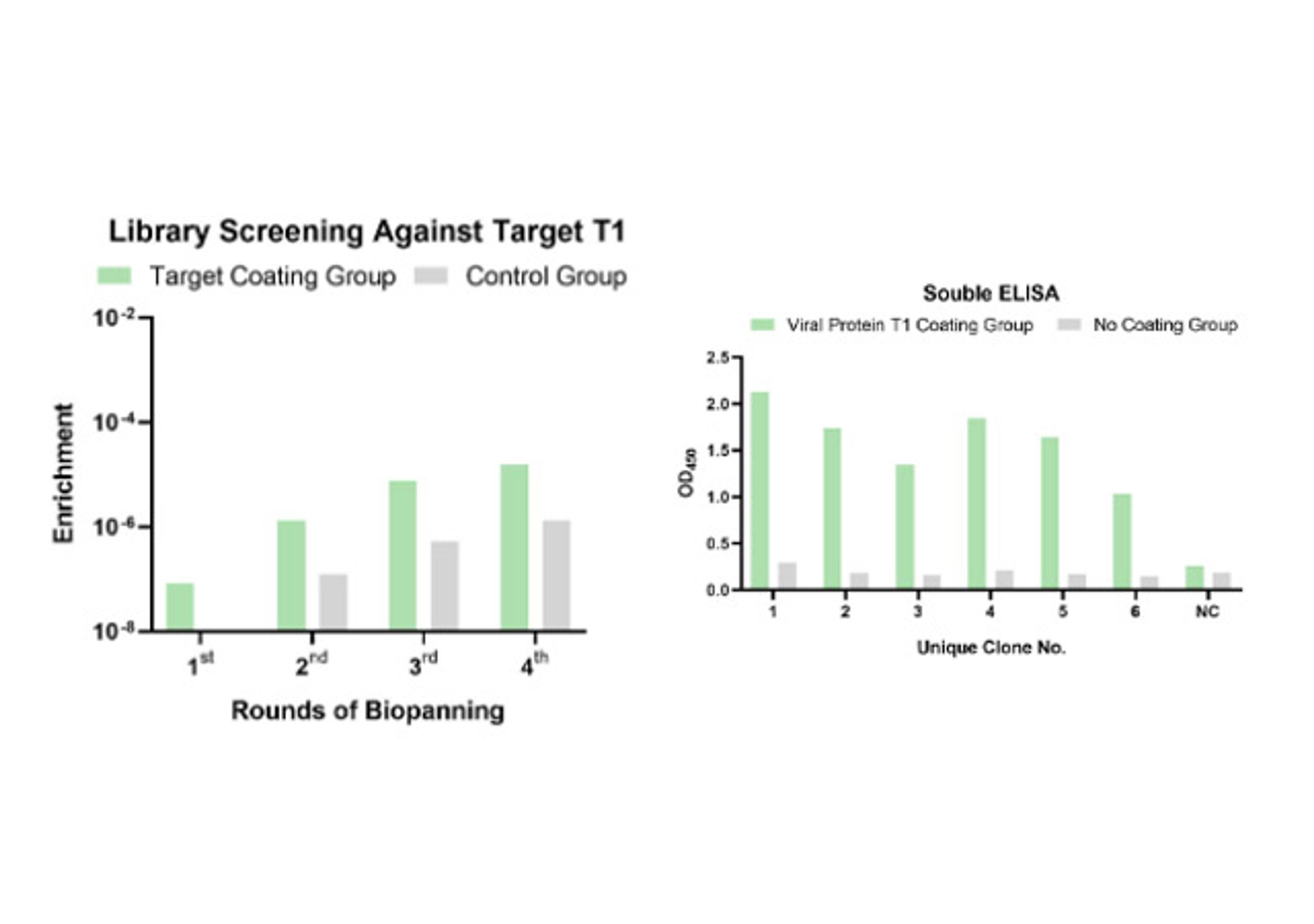
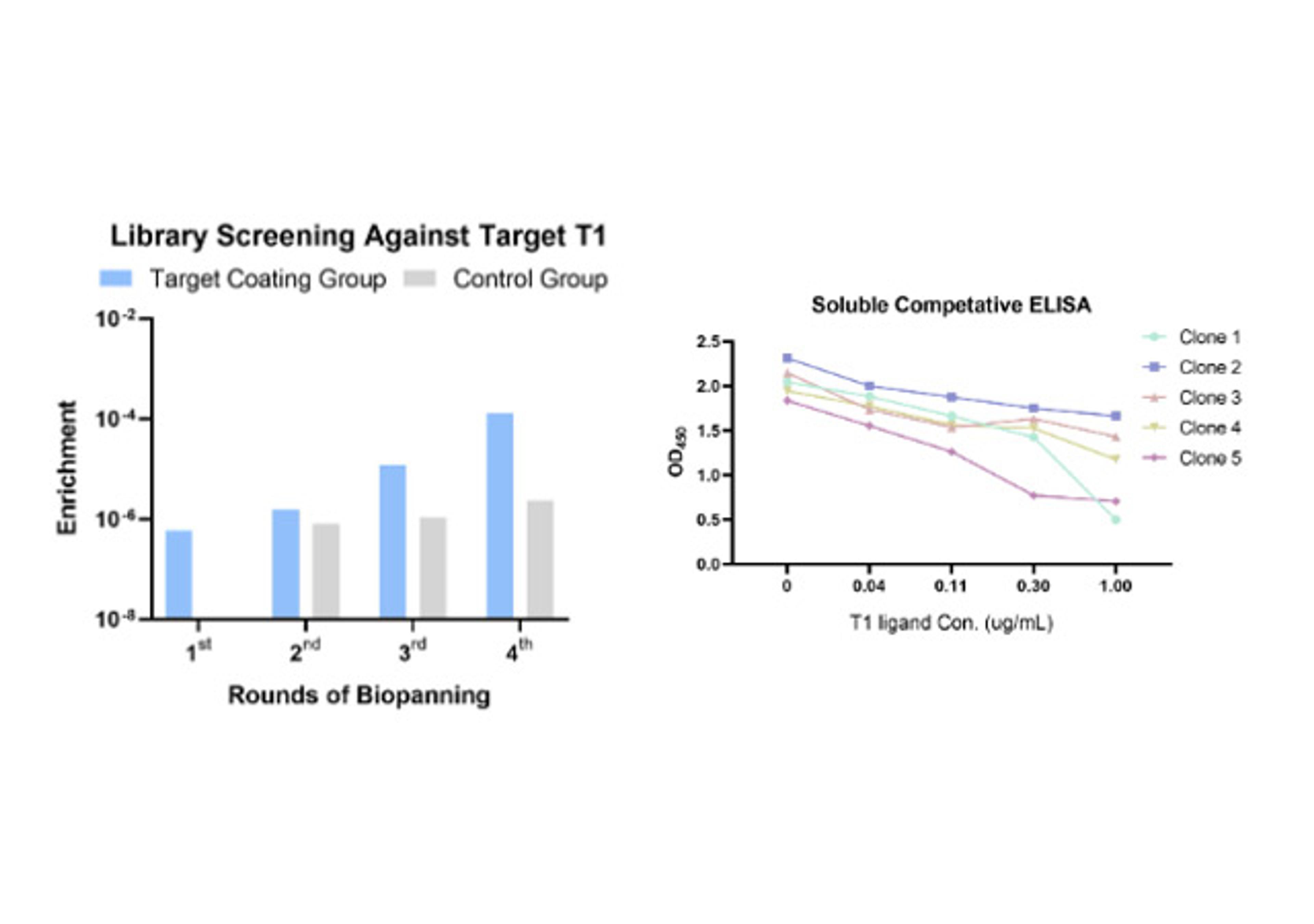
- In vitro display (Phage & Yeast): These platforms are engines of pure numbers. By screening vast libraries—often exceeding 108-1011 unique variants—Creative Biolabs performs "panning" against purified targets. This in vitro approach gives it total control. It can select for binders that target a specific domain, or "compete" with a known ligand to isolate functional antibodies that block or mimic its action.
- In vivo maturation (Hybridoma & B-cell sorting): This is nature's gold standard. When an animal is immunized, its immune system performs a selection process of unparalleled rigor, maturing B-cells to produce antibodies with exquisite affinity and specificity, while simultaneously eliminating self-reactive clones. Modern Single B Cell Sorting technology now allows us to bypass the slower hybridoma process, directly isolating and sequencing the antibody genes from promising B-cells in just days.
Pillar 3: Choosing the right format for the job
Often, a conventional 150 kDa IgG is simply the wrong tool. Its large size physically prevents it from accessing the most valuable targets: the "cryptic epitopes" buried within the functional clefts of receptors.
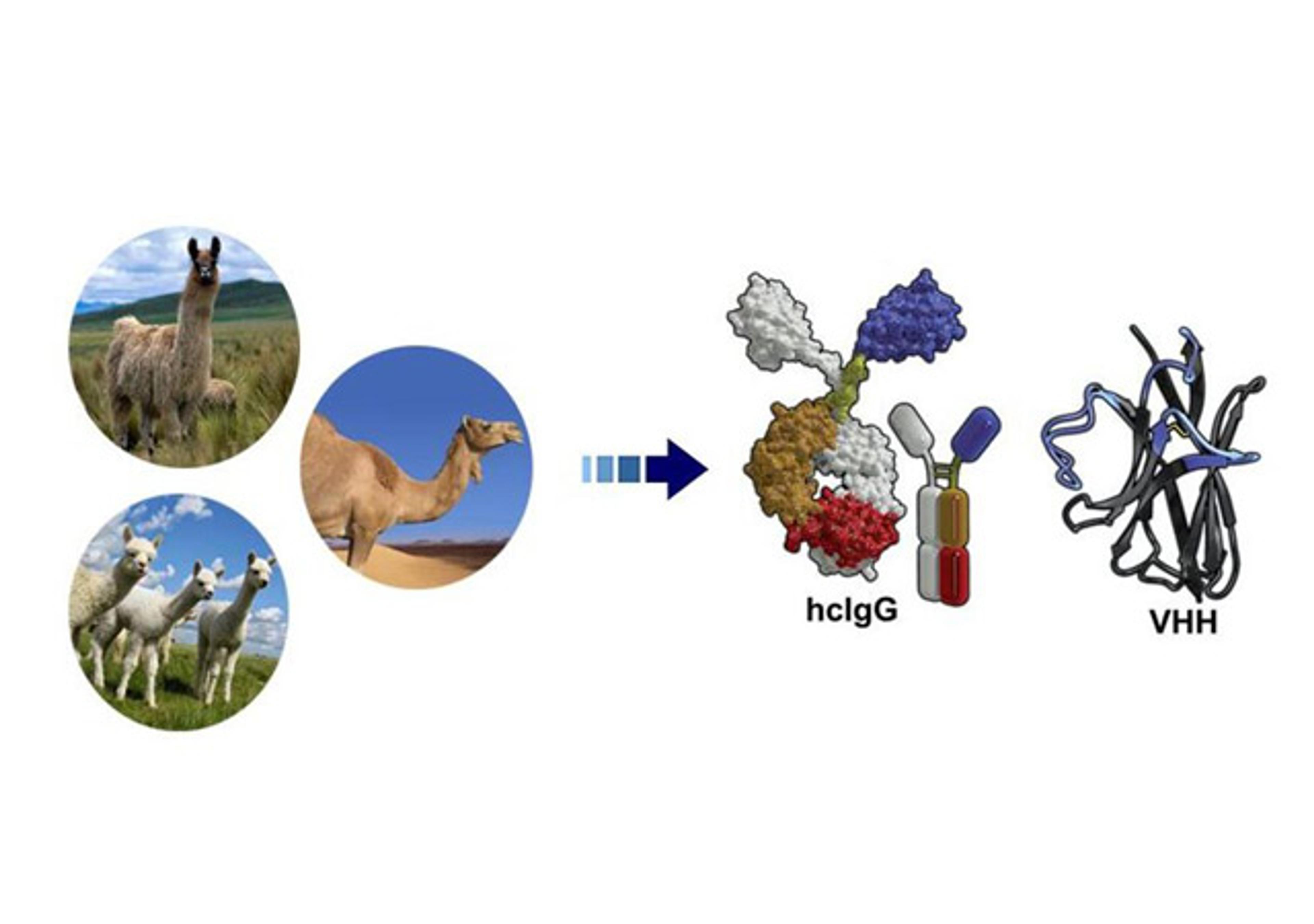
This is where Single Domain Antibodies (SdAbs) have become game-changers. Derived from camelids, these tiny ~15 kDa fragments possess a unique, long CDR3 loop. This loop acts like a "finger," allowing it to reach deep into the active sites of GPCRs or the pores of ion channels. This is what enables the creation of true functional modulators—agonists and antagonists—that were previously impossible to generate with traditional mAbs.
The future: The 'druggable' target list is growing
The "undruggable" status of membrane proteins is fading. The key to this new frontier is not just having these technologies but having the deep expertise to integrate them. The right strategy may be immunizing with VLPs, followed by B-cell sorting to find a human IgG. For another target, it might be screening a phage display library of SdAbs against a nanodisc-stabilized GPCR. This sophisticated integration—matching the right antigen preparation method with the right discovery engine and the right antibody form—is how Creative Biolabs consistently tackles some of the industry's most challenging problems. As Creative Biolabs refines these integrated solutions, it's not only discovered antibodies, but it's also opened doors to entirely new drug classes.