Organoids offer new dimension to aging research
See how the team of Simone Sidoli is using organoid technologies to model aging in a lab
5 Jan 2026
3D cell cultures – particularly organoids – are emerging as a more physiologically relevant alternative to traditional 2D approaches. These miniature, lab-grown structures replicate the architecture and function of human organs far more accurately, offering a more translational and personalized model system for research.
Among the scientists turning to 3D approaches is Dr. Simone Sidoli, Assistant Professor at the Albert Einstein College of Medicine, who is using organoid technology to study the science of aging. While aging is an apparent phenotype in humans, its underlying cause is still unknown and Dr. Sidoli aims to understand such mechanisms through a 3D lens.
Aging is inherently a 3D process; it affects not just molecular pathways but also cellular architecture, tissue structure, and mechanical interactions. Organoids offer a unique window into these dynamics. Unlike monolayer cultures, which flatten cellular behavior into two dimensions, organoids preserve the spatial organization necessary to study how chromatin decondensation unfolds in real tissue-like environments.
Find the latest 3D cell culture news in our Accelerating Science Feature exploring how scientists are uncovering the mechanisms behind disease and driving breakthroughs in treatment.
Visit resourceOrganoids reveal new mechanisms behind human aging
One project of the Sidoli Lab explores how protein modifications, particularly histone succinylation, contribute to genomic stability in models of exceptional longevity. Disruption of chromatin structure is a characterized phenomenon during aging, leading to transcriptional dysregulation and cellular dysfunction. Their findings suggest that succinylation – a modification more prevalent in individuals with projected exceptional longevity – may help preserve chromatin integrity by preventing transcription factor binding and inhibiting lysine demethylases. This inhibition promotes the formation of compact heterochromatin, potentially shielding the genome from age-related damage and pointing to new therapeutic strategies for healthy aging.
An area of particular interest is the modeling of this chromatin decondensation without affecting the proliferation rate of cells. This is a nuanced challenge, one that requires cellular environments that closely resemble those in vivo. “In a 2D plate, cells often behave abnormally; their gene expression profiles can diverge significantly from what we observe in actual human tissues,” he notes. “With organoids, we can maintain both cellular architecture and functionality, allowing us to study phenomena like chromatin dynamics in a more biologically relevant context.”
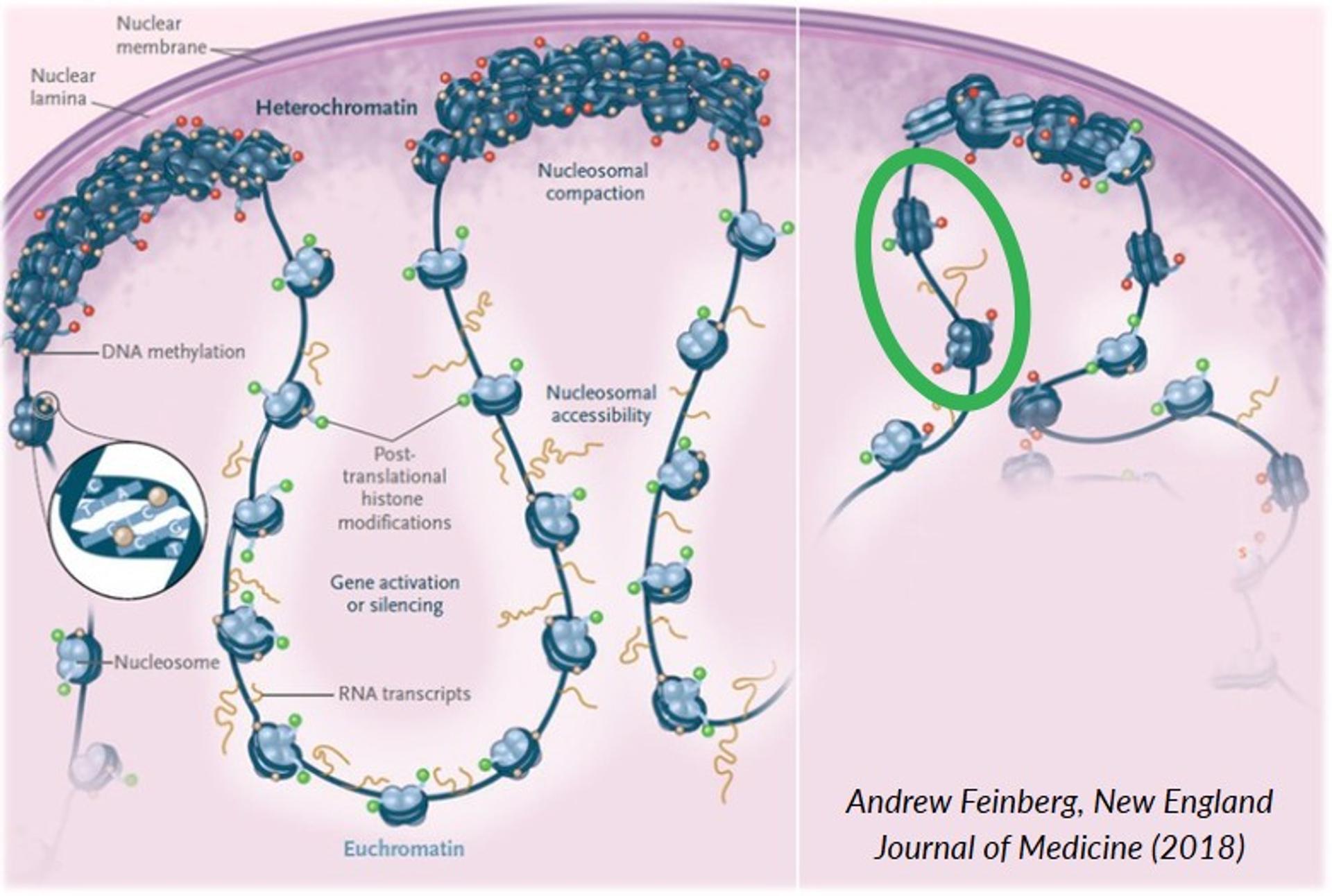
During aging, chromatin loses condensation and DNA readout becomes dysregulated leading to cell dysfunction. Identifying molecules that “protect” open chromatin domains from anomalous transcriptional regulation would lead to new therapeutic avenues. The Sidoli Lab’s goal is to identify these protein modifications that protect chromatin.
Moreover, Dr. Sidoli explores the concept of biological replicates in a way that redefines standard lab practices. Traditional experiments often rely on replicates from different plates or wells, introducing variability based on subtle differences in handling or surface area. With organoids, each structure becomes a self-contained replicate, more accurately representing biological individuality. This shift enhances the statistical and experimental power of studies, especially in complex fields like neuroscience or oncology, where reproducibility is paramount.
ClinoStar powers advanced 3D cell culture workflows
For such delicate and demanding research, the culture system must be equally advanced, which is where the ClinoStar system proves invaluable. Developed by CelVivo, the ClinoStar is a standalone incubator designed specifically for 3D cell culture. Its key innovation lies in its unique bio-reactor ClinoReactor utilizing the clinostat principle. Six independent bio-reactors are controlled in the ClinoStar allowing researchers to fine-tune the rotation speed of each reactor. This not only supports the formation of uniform organoids but also mimics the microgravity environment that encourages natural tissue-like growth.
ClinoStar’s environmental controls are equally sophisticated. Researchers can remotely manage temperature, CO₂ levels, rotation speeds, and optionally O2-levels, through the CelVivo Cloud interface, allowing for seamless experimental continuity. For Dr. Sidoli, this feature has improved both the consistency of his results and the flexibility of his workflow. “I can make adjustments without needing to physically be in the lab, which is especially helpful for long-term cultures or when coordinating with collaborators in different time zones,” he explains. Notably, the Sidoli lab is the designated United States Center of Excellence for CelVivo.
The inclusion of six individual cameras offers real-time visual feedback, giving researchers comprehensive oversight of their experiments without the need to open the chamber and risk contamination. Small design features, like a push-to-open door for hands-free operation and a built-in whiteboard for notes, speak to how thoughtfully the system is tailored for real-world lab environments.
Importantly, the system’s UV-C decontamination beacon adds an additional layer of sterility, which is vital when working with sensitive 3D cultures. Altogether, these features contribute to a more controlled, efficient, and hygienic workspace – elements that often go underappreciated but are crucial for research that demands precision.
A platform for personalized medicine and beyond
The broader promise of organoid research lies in its ability to usher in an era of personalized medicine. Because organoids can be derived from patient-specific stem cells, they allow researchers to model disease and treatment responses on an individual level. In cancer research, for instance, tumor organoids have been used to test chemotherapy drugs ex vivo, enabling clinicians to tailor regimens based on a patient’s actual tumor biology rather than population-level averages.
As interest in high-throughput drug screening grows, the importance of having reliable, reproducible culture systems becomes even greater. Systems like ClinoStar and its companion, the ClinoReactor, make it easier to scale up organoid research. The ClinoReactor’s petri dish-style access and stable gas exchange capabilities mean large tissue constructs can be handled with minimal disruption and maximum reproducibility.
This scalability is particularly relevant in pharma, where drug discovery and development pipelines demand hundreds, if not thousands, of data points across multiple conditions. Traditional bioreactor systems often fall short in ease of access, contamination control, or environmental consistency. ClinoReactor addresses these pain points with a self-supporting design, integrated humidification, and simplified media exchange via vials.
Where innovation meets biology
Organoid research is at a tipping point. A 2023 review in Nature Reviews Molecular Cell Biology emphasized the growing role of 3D culture systems in recapitulating human development and disease, particularly highlighting their capacity to model tissue-tissue interactions and cellular heterogeneity – both critical for translational research. As the field matures, technologies like ClinoStar and ClinoReactor will be essential not only in enabling complex biology but in making it accessible and routine.
For researchers like Dr. Sidoli, the combination of advanced 3D cell culture systems and innovative incubator design is opening up new frontiers in aging science. More importantly, it’s changing the way science is done by making labs more efficient, more productive, and better suited to handle the complexity of modern biomedical challenges.
As more researchers turn to organoids for their experimental models, having tools that support not just cell growth but scientific growth will be key. In that sense, the ClinoStar isn’t just an incubator, it’s a catalyst for progress.

