Accelerating targets and modalities – how cryo-EM is transforming drug discovery and development
In this guest editorial article, learn how scientists are leveraging cutting-edge cryo-EM workflow advancements and their impact in drug discovery
5 Sept 2022
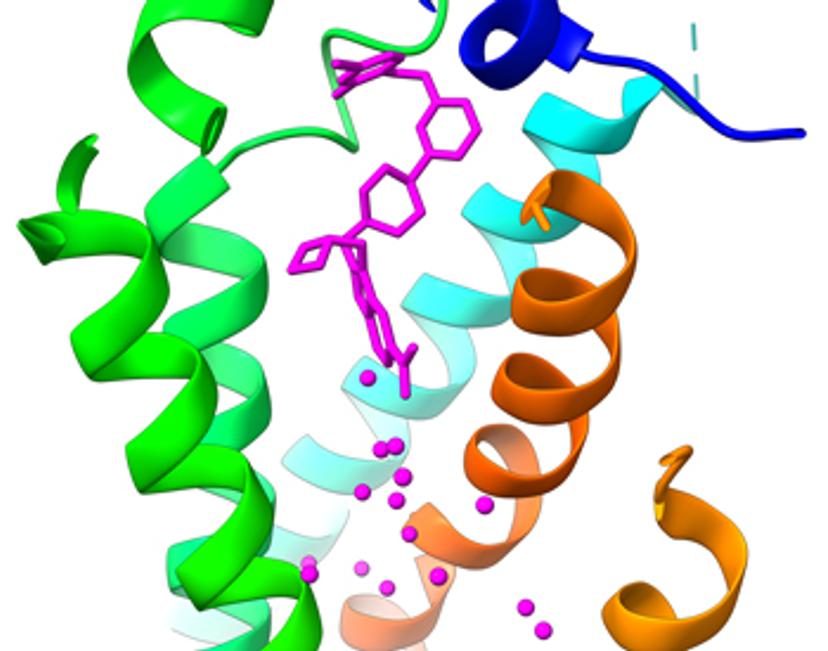
Cryo-electron microscopy (cryo-EM) is a structural biology technique that has become an invaluable tool for drug discovery by bringing the benefits of rational structure-based drug design to challenging targets. The technique has underpinned success for many small molecule and biologics programs that have reached clinical stages of development and beyond. Behind these programs lie significant cryo-EM innovation that have allowed more companies than ever to diversify, accelerate, and de-risk discovery and development pipelines.
Thermo Fisher Scientific recently announced a collaboration with Structura Biotechnology that combines the power of Thermo Scientific™ EPU Multigrid Software and Structura’s CryoSPARC Live. The result is a dramatic reduction in the time it takes to transform raw data into high-quality 3D protein structures from days to hours.
Cryo-EM is most powerful when it moves from a supporting role to become a central driver of a drug discovery program, which can be achieved by tightly integrating the technique with other experimental or computational platforms. As part of the SelectScience® Advances in Drug Discovery Special Feature, in this guest editorial, Mazdak Radjainia, Ph.D., Senior Staff Scientist, Cryo-EM SBDD collaborations and platform development, Thermo Fisher Scientific, presents several case studies that leverage innovative workflow advancements and external technologies as well as their impact in drug discovery.
High-throughput cryo-EM epitope mapping of SARS-CoV-2 spike protein antibodies with Utrecht University and Takeda Pharmaceuticals
Therapeutic antibodies targeting the SARS-CoV-2 spike protein can play an important role in reducing the burden of the COVID-19 pandemic by protecting vulnerable populations against severe and potentially life-threatening disease. One of the main concerns is SARS-CoV-2 variants that may be able to evade immunity and escape the actions of therapeutic antibodies. Epitope mapping of large antibody panels allows researchers to identify vulnerable sites on the spike protein, predict and interpret the effect of new mutations, and speed up decision-making in selecting antibody combinations that target non-overlapping epitopes. Moreover, structural information about epitopes can help identify liabilities in drug discovery pipelines in response to new emerging variants.
In a recent collaboration, Utrecht University and Takeda Pharmaceuticals worked together to find out whether cryo-EM permits rapid epitope mapping for a large antibody panel. Indeed, using a new data acquisition mode for Thermo Scientific autoloader-equipped cryogenic transmission electron microscopes (cryo-TEMs) allowed them to obtain 12, sub-3 Å structures of the SARS-CoV-2 spike protein in complex with RBD-binding Fabs, and to determine their epitopes. Their results, which are described in a white paper, show that cryo-EM is now poised to emulate the high-throughput revolution of macromolecular crystallography thanks to dramatic improvements in data collection.
High-throughput cryo-EM epitope mapping of SARS-CoV-2 spike protein antibodies using Thermo Scientific™ EPU Multigrid Software. Spike-Fab complex grids were vitrified in duplicate and screened using the Thermo Scientific™ Glacios™ Cryo-TEM. Twelve best grids (one grid per complex) were taken forward for automated acquisition using the Thermo Scientific™ Krios™ G4 Cryo-TEM and EPU Multigrid Software. 48-hour EPU Multigrid session resulted in twelve sub 3 Å cryo-EM reconstructions that allowed for mapping of epitope-paratope residues, as well as assignment of Fabs to the epitope classes.
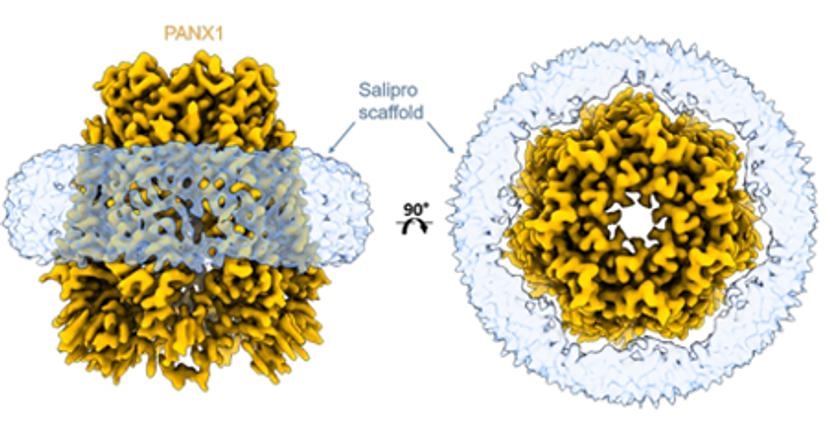
Racing to structure-enabled hits with Salipro Biotech
Drug discovery programs move the fastest when promising hits can be identified sooner and the quicker they can be optimized to be potent and safe enough for clinical investigation. To maintain aggressive timelines, it is critical to swiftly generate high-quality, pure protein for binding assays and structure determination. Likewise, early structure enablement allows medicinal chemists to work with high precision from the outset, and to advance compounds quickly through hit to lead as well as early lead optimization phases. However, membrane protein targets are notoriously challenging to produce, difficult to immobilize for binding assays, and, due to the presence of detergents, artifact-prone.
A recently published preprint with Salipro Biotech and AstraZeneca describes a streamlined blueprint for producing membrane proteins and successfully subjecting them to surface plasmon resonance (SPR) and cryo-EM. The approach is based on the Salipro DirectMX method that can be used to reconstitute membrane proteins directly from crude membranes. Salipro DirectMX yields purified membrane proteins that can be either imaged by cryo-EM or tethered for SPR without interfering mutations and detergents. In other words, it showed that the journey from cell pellet to screening compounds by SPR, as well as visualization of their interaction with the membrane protein target, can be performed in a very short time.
Cryo-EM processing at the pace of medicinal chemistry on Amazon Web Services
As noted in the two sections above, delivering timely structures is key to guiding critical medicinal chemistry decisions. This requires that multiple compound-bound structures can be turned around in a matter of days and at resolutions better than 3 Å, making it important to compress timelines of cryo-EM data processing from weeks to days, if not hours. Furthermore, to increase the accessibility of cryo-EM it is important to reduce IT barriers. A collaboration with Amazon Web Services (AWS) explored cloud-based high-performance computing (HPC) for cryo-EM. It showed that for prototypical drug discovery projects, structures can be obtained in hours to a day on AWS. To do so, an AWS service called ParallelCluster was used, which deploys powerful HPC clusters on demand. AWS provides turn-key solutions for cryo-EM processing that have excellent performance and which scale with the needs of users. The result is that better structures can be obtained in lockstep with medicinal chemistry cycles.
Conclusions
The three examples above demonstrate how cryo-EM is becoming a central driver in the design of better molecules in shorter time frames. Improvements span the entire workflow with a strong trend towards dramatically reduced timelines for every step from protein production to data collection. This allows cryo-EM to connect with the most impactful phases of molecule screening campaigns or computationally driven drug design, including AI-driven drug design. Thus, efficient integration of cryo-EM with other experimental or computational platforms helps researchers reimagine the design of highly potent and safe medicines.
Visit the SelectScience Advances in Drug Discovery Special Feature to learn more about the latest technologies and methods transforming the industry.


