Search results for "Rigaku Analytical Devices"
Selected Filters:
New Device Concentrates Protein from Single Drop
TM-1000 Boost For Medical Device Imaging
PMA-Lite™ 2.0 LED Photolysis Device
BiotiumAn optimized photoactivation device designed for photolysis of PMAxx™- and PMA- treated samples in viability PCR.
AXIS™ Axon Isolation Device, 150 µm
MerckAXIS devices physically isolate developing neurites from each other and their respective neural cell bodies. In addition, the use of hydrostatic pressure during analysis provides researchers with an opportunity for targeted exposure of neuronal parts to growth factors, drug compounds, or other reagents of biological interest. This ability to selectively control exposure of neuronal outgrowth makes AXIS a powerful platform for…
IMAG-12T Hand-Held Magnetic Separation Device
Corning Life SciencesThe use of paramagnetic bead based separation devices (MSDs) are an essential accessory in any magnetic bead based purification process. However, traditionally MSDs are not optimized for manual use and most require electrically powered liquid handling systems. The IMAG MSDs in its tube or microplates formats are designed to simplify the manual processing of magnetic bead based separation applications including: Nucleic acid pu…
IMAG-96P Hand-Held Magnetic Separation Device
Corning Life SciencesThe use of paramagnetic bead based separation devices (MSDs) are an essential accessory in any magnetic bead based purification process. However, traditionally MSDs are not optimized for manual use and most require electrically powered liquid handling systems. The IMAG MSDs in its tube or microplates formats are designed to simplify the manual processing of magnetic bead based separation applications including: Nucleic acid pu…
Tasso+™ Device and Tasso+™ Kits
Tasso, Inc.Patient-centric device and kits for collecting whole liquid blood samples
Wildcat Laboratory Solutions
Wildcat Laboratory Solutions is a trusted provider of laboratory automation equipment, consumables, and services, delivering tailored solutions across the UK and EU. The company specializes in supporting cutting-edge research with innovative, scalable, and customer-focused solutions.
SQL Anywhere
SybaseSQL Anywhere is a comprehensive suite of solutions that provides data management, synchronization and data exchange technologies that enable the rapid development and deployment of database-powered applications in remote and mobile environments. Developers can use the database and synchronization technologies in SQL Anywhere to power data-driven applications in server, mobile and remote workgroup environments. SQL Anywhere sup…
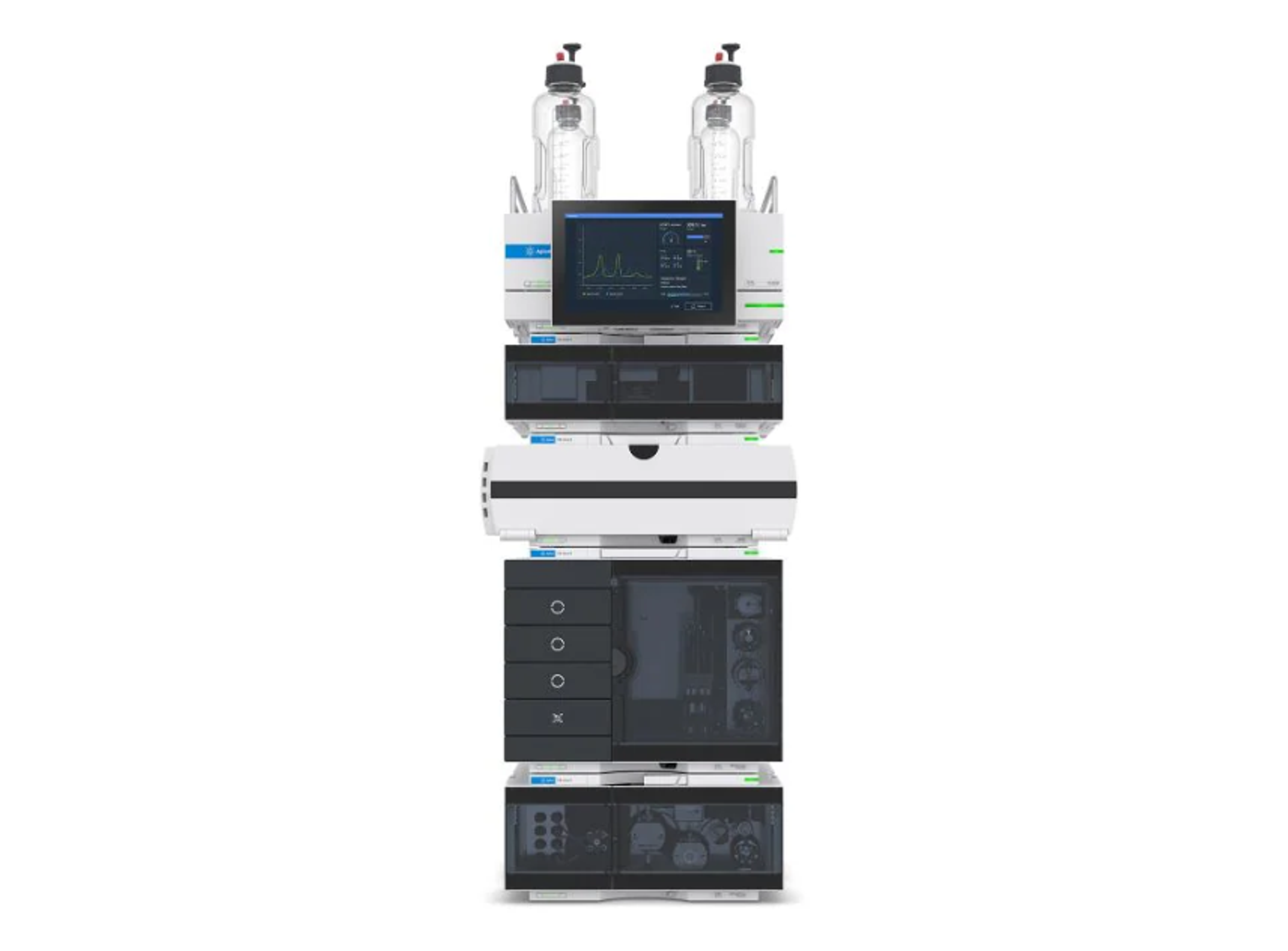
Agilent 1260 Infinity III Prime LC System
Agilent TechnologiesThe 1260 Infinity III Prime LC System is an analytical ultrahigh-performance liquid chromatography instrument. It operates at high pressures and can handle flow rates up to 5 mL/min for using UHPLC columns packed with sub-2 μm particles. This HPLC/UHPLC system gives you the utmost confidence in your HPLC results and is a perfect front end for your LC/MS system.
SelexION differential mobility spectrometry
SCIEXSelexION differential mobility spectrometry is an effective ion mobility spectrometry tool for improving data quality in the quantitation and characterization of challenging samples requiring advanced analytical selectivity. SelexION on the SCIEX 5500+ series and 6500+ series delivers a new dimension of selectivity and performance for applications requiring the separation of isobaric species, elimination of challenging co-elut…
ultraWAVE SRC (Single Reaction Chamber) Microwave Digestion System
MilestoneultraWAVE has already revolutionized and enhanced the way analytical chemists think to sample preparation for trace metal analysis in hundreds of laboratories all over the world. ultraWAVE is based on the SRC (Single Reaction Chamber technology), invented by Milestone several years ago ( EP0728038B1 ). SRC technology achieves extraordinary performance capabilities combining microwave heating with a high-pressure reactor which…
PURELAB® Option-Q
ELGA LabWaterUltra pure water direct from tap water The PURELAB Option-Q has been developed by ELGA to produce ultra pure (type I) water without the need for pre-purified feed. It can deliver up to 15 liters per hour (100 liters per day) of 18.2MΩ-cm water direct from a portable supply. This makes the PURELAB-Q the cost-effective choice for lifescience, analytical, and general applications.
NucleoCounter® NC-3000™ advanced cell analyzer
ChemoMetecThe NucleoCounter® NC-3000™ is an advanced image cytometer utilizing fluorescence imaging to characterize cell properties. The NC-3000™ can perform high-speed cell count and viability analyses and it includes plug-and-play analytical assays of mammalian, yeast and insect cells. Pre-defined assays include a unique 5-minute cell cycle assay, a selection of apoptosis assays and a GFP transfection efficiency assay.
Custom Immunoassay Menu of Services
IR Spectroscopy Defines Catalysed Reactions
Arrayjet Launches Custom Microarray Services
Waters Educational Services 2007 Catalog
Custom Nucleic Acid Production Services
Creative BiolabsCustom DNA/RNA oligonucleotide synthesis with broad modification choices (PS, 2′-OMe, 2′-F, LNA, GalNAc, dyes/biotin), multiple scales and purifications, and analytical QC. Includes design consultation and documentation to support research, translational studies, and conjugation-ready formats.
Protein Expression and Purification Services
Creative BioMartEnd-to-end custom services for the cloning, expression, and purification of soluble recombinant proteins in various host systems, from gene synthesis to purified protein delivery.