Immunology Products & Reviews
Immunological techniques measure and characterize immune responses. Immunology kits and analysis systems often use techniques such as ELISA, radioimmunoassay (RIA) and immunodiffusion assays, Immunohistochemistry, and flow cytometry. Immunologists use equipment such as flow Cytometers, plate readers, plate washers and fluorescent microscopes.
Selected Filters:
Tissue Samples for Research
The Niche CRO GroupTissue Solutions is a leading global provider of ethically sourced human tissue samples used in all areas of drug discovery, research and development. ISO 9001:2015 certified and twice winner of the coveted Queens Award for Enterprise, Tissue Solutions offer a single point of access for a wide range of diseased and normal tissues in fresh, frozen, and FFPE formats.
Histology Contract Services
The Niche CRO GroupHistologiX is a leading GLP/GCP accredited Contract Research Organisation based in the UK specialising in Histology, Immunohistochemistry (IHC) and Digital Pathology services. HistologiX works with a broad range of clients from big pharma to small and medium biotech companies worldwide. Our team of scientists have in excess of 120 years combined experience and a customer retention rate of 80%.
Immunohistochemistry Services
The Niche CRO GroupHistologiX is a leading GLP/GCP accredited Contract Research Organisation based in the UK specialising in Histology, Immunohistochemistry (IHC) and Digital Pathology services. HistologiX works with a broad range of clients from big pharma to small and medium biotech companies worldwide. Our team of scientists have in excess of 120 years combined experience and a customer retention rate of 80%.
C083 Cellulose Fiber Sample Pad Sheets
MerckC083 Cellulose Fiber Sample Pad Sheets for use in lateral flow, assay development, lateral flow immunoassays, lateral flow assay devices & immunoassay IVDs
PCRD & PCRD FLEX
Abingdon HealthRapid nucleic acid detection post isothermal amplification for decentralised, point of care molecular testing
Gibco™ CTS™ Rotea Counterflow Centrifugation System
Thermo Fisher ScientificCompact, closed cell processing system designed to streamline and expedite your cell therapy development.
FO-SPR Carboxyl probes
FOx BiosystemsDip-in probes with a generic surface chemistry that can couple a wide range of biomolecules
Bio-Plex Multiplex SARS-CoV-2 Serology Assay Kits and Reagents
Bio-RadProvides sensitive and specific measurements of antibodies against four SARS-CoV-2 proteins: nucleocapsid, receptor binding domain, spike 1, and spike 2 viral proteins.
KeyPro™ KP100 UV LED Decontamination System
Phoseon TechnologyUV LED decontamination using Phoseon’s KeyPro™ KP100 instrument benefits labs that require high fidelity RNA library prep, sequencing and PCR, or for vaccine development labs that need to quickly and reliably inactivate viruses.
Bio-Plex Multiplex SARS-CoV-2 Serology Assays
Bio-RadProvides sensitive and specific quantitation of antibodies against four SARS-CoV-2 proteins: nucleocapsid, receptor binding domain, spike 1, and spike 2 viral proteins.
InteliQ Homocysteine Control
Bio-RadInteliQ Homocysteine Control is a ready-to-use quality control in a barcoded tube designed to monitor the precision of homocysteine test procedures.
InteliQ Immunoassay Plus Control
Bio-RadInteliQ Immunoassay Plus Control is a ready-to-use quality control in a barcoded tube designed to monitor the precision of immunoassay and therapeutic drug monitoring tests.
InteliQ Immunology Control
Bio-RadInteliQ Immunology Control is a ready-to-use quality control in a barcoded tube designed to monitor the precision of immunology assays.
InteliQ Specialty Immunoassay Control
Bio-RadInteliQ Specialty Immunoassay Control is a ready-to-use quality control in a barcoded tube designed to monitor the precision of specialty immunoassay testing. This control includes additional complex analytes and is designed to complement Bio-Rad's Immunoassay Plus controls.
InteliQ Tumor Marker Control
Bio-RadInteliQ Tumor Marker Control is a ready-to-use quality control in a barcoded tube designed to monitor the precision of immunoassay based oncology testing.
VIROTROL SARS-CoV-2 (CE/IVD Mark)
Bio-RadAn unassayed, reactive quality control for the qualitative determination of total IgG/IgM and IgG antibodies to SARS-CoV-2.
VIROCLEAR SARS-CoV-2 (CE/IVD Mark)
Bio-RadAn unassayed, non-reactive quality control for the qualitative determination of total IgG/IgM and IgG antibodies to SARS-CoV-2.
Liquichek Immunology Control
Bio-RadA trilevel, liquid, comprehensive serum protein control with assayed values for a wide range of methods; includes Anti-Cyclic Citrullinated Peptide (Anti-CCP) and Rheumatoid Factor (RF).
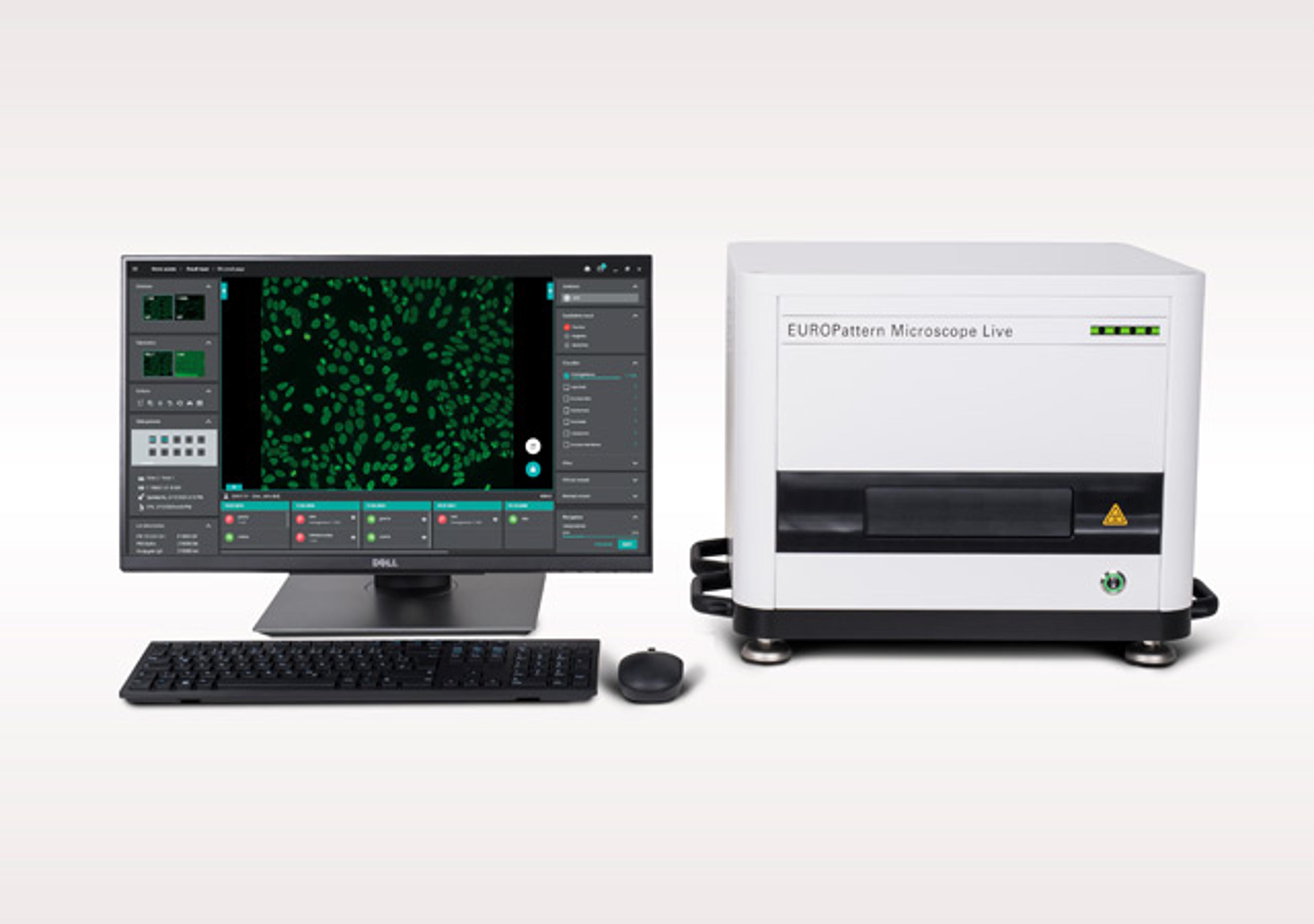
EUROPattern Microscope Live
EuroimmunThe EUROPattern Microscope Live provides fully automated immunofluorescence microscopy with generation of result proposals using AI-enhanced software. It has a processing time of only 2 seconds per image due to automatic laser focusing. The EUROPattern Classifier provides positive-negative discrimination, pattern recognition and titer determination for many applications. Results are consolidated into one proposal per patient.
Calprotectin Assays
EuroimmunEuroimmun offers ChLIA and ELISA tests for fast and efficient measurement of faecal calprotectin in stool samples. The analysis supports the diagnostic differentiation between chronic inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS) in line with current international guidelines. The assays are fully automatable, with random-access processing available for the ChLIA.