Clinical Diagnostics Products & Reviews
Clinical diagnostics encompasses the detection, diagnosis, and monitoring of diseases and health conditions in patients. Explore how this field empowers clinicians to deliver accurate diagnoses and improve patient outcomes with the latest clinical diagnostics news, technology advances, expert webinars, and product reviews curated by SelectScience.
Selected Filters:
Color-coded MC-Media Pads®
Merck KGaA, Darmstadt, GermanyMC-Media Pad® convenient culture media is used for rapid routine testing of microbial contamination in food and beverage samples throughout production, from raw materials, to finished products. They are based on chromogenic substances included in a media layer to give faster results while reducing the need for time-consuming agar media preparation time. Simply pipette 1 mL of sample to the center of the pad—it diffuses automat…
EP36
Clinical and Laboratory Standards InstituteHarmonization of Symbology and Equations, 1st Edition
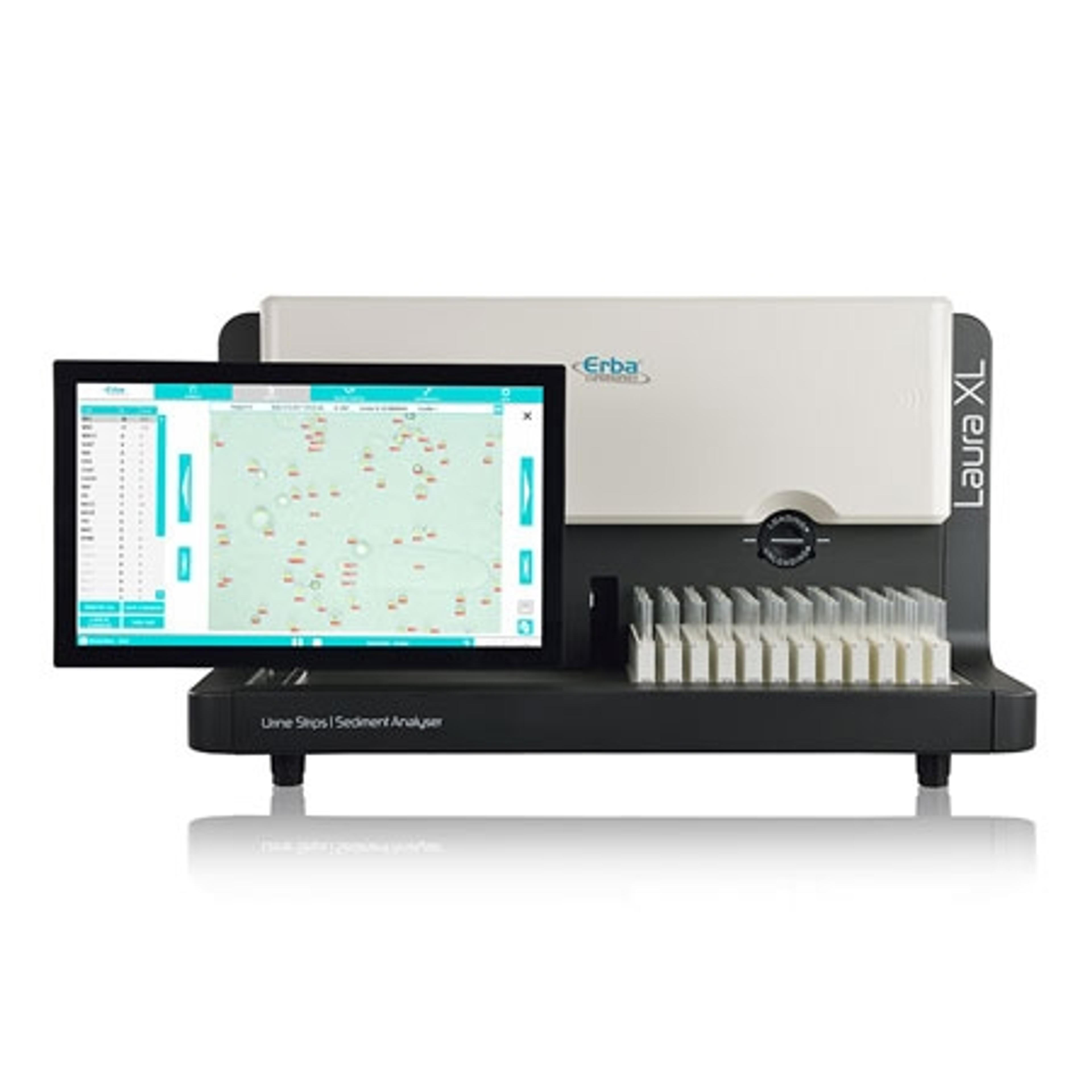
Biochemical analyzer XL 640
Erba MannheimXL 640 is a fully automated biochemistry analyzer with a throughput of 640 tests per hour. It is a dependable, powerful and robust analyzer to keep up with the heavy demands of everyday. XL 640 is designed to operate smoothly over the long haul and to deliver consistent quality report.
Biochemical analyzer XL 1000
Erba MannheimXL 1000 with the throughput of upto 1040 tests per hour is the ideal instrument for centralizing the complete biochemistry testing load on a single fully automated platform. Increased walk away productivity and less human intervention make it an apt analyzer for large laboratories.
Nanotrap® Virus Capture Kit (50)
Ceres Nanosciences, Inc.The Ceres Nanosciences Nanotrap® Virus Capture Kit provide a way to concentrate viruses from complex biological matrices in order to have high quality input material for your downstream analytical methods
Nanotrap® Technology Development Projects
Ceres Nanosciences, Inc.Collaborate with Ceres Nanosciences to optimize the Nanotrap® technology platform for the capture, concentration, or preservation of your valuable analytes and pathogens.
µPAC™ Grounding Cable
PharmaFluidicsBecause the column is meanly made out of silicon which is semi-conductive, a dramatic impact on the chromatographic behavior of our µPAC™ columns has been observed if no appropriate precautions are taken in case the column is connected to an ESI source of a mass spectrometer. Therefore we developed a custom made grounding cable to shunt the high voltage to ground.
µPAC™ capLC column
PharmaFluidicsThe µPAC™ capLC columns are designed for increased robustness and throughput assuring sensitivity. A flow rate versatility between 1 and 15 µL/min at moderated pressures allows short gradient separations, while the µPAC™ technology ensures an exceptional high reproducibility over time and across laboratories. µPAC™ column, 50cm length, with pillar array backbone at interpillar distance of 2.5 µm, C18.
QIAamp MinElute ccfDNA Mini Kit (50)
QIAGENFor isolation of free-circulating DNA from human plasma or serum
CryoPod™ Filling Station
Brooks Life SciencesThe CryoPod™ Carrier Filling Station enables the fast and simple replenishing of the CryoPod™'s LN2 supply in a safe, precise, and hands-free manner
BioStore™ IIIv Automated Storage System
Brooks Life SciencesBioStore™ IIIv is an automated LN2 alternative to electromechanical upright -80 freezers. Consistent temperature, sample protection and onboard electronic inventory provides detailed inventory records.
Thermo Scientific™ Nunc™ UpCell cultureware
Thermo Fisher ScientificCell Culture Plates and dishes, available in numerouse formats
Thermo Scientific™ Nunc™ Cell Culture Inserts
Thermo Fisher ScientificNunc™ Polycarbonate Cell Culture Inserts
therascreen FGFR RGQ RT-PCR Kit
QIAGENtherascreen FGFR RGQ RT-PCR Kit - For qualitative detection of actionable alterations in the FGFR3 gene.
PD-L1 IHC 22C3 pharmDx for Autostainer Link 48
Dako North America, Inc.PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in certain types of formalin-fixed, paraffin-embedded (FFPE) cancer tissues using EnVision FLEX visualization system on Autostainer Link 48.
ABL9 blood gas analyzer
RadiometerYour blood gas analyzer designed for easy blood gas testing at the point of care
ABL80 FLEX CO-OX OSM blood gas analyzer
RadiometerWhen simplicity and quality CO-oximetry is paramount, consider the ABL80 FLEX CO-OX analyzer–OSM version, which delivers reliable test results on CO-oximetry parameters in as little as 60 seconds
AQURE point-of-care IT Solution
RadiometerGet a complete overview of your point-of-care testing setup with AQURE IT solution
HybridSPE®-Plus Plates
MerckOur HybridSPE ® -PLus plates effectively remove phospholipids and proteins from biological matrices in a simple pass-through method for accurate and reproducible LC-MS analysis. Unlike other phospholipid removal SPE products on the market, the unique retention mechanism of HybridSPE ® allows for separation of even very hydrophobic analytes from phospholipid contaminants.
Corning® 2 mL External Threaded Polypropylene Cryogenic Vial with 1D and 2D Bar codes
Corning Life Sciences2 mL capacity with round bottomUnique 1D, 2D barcodes and human readable charactersManufactured from polypropylene to withstand temperatures to -196°CCaps designed for a secure seal and compatible with automatic decapping equipmentLocking baseSterile SAL 10⁻⁶Certified nonpyrogenic and DNase/RNase-free
Corning® 2 mL External Threaded Polypropylene Cryogenic Vial with 1D Barcode
Corning Life Sciences2 mL capacity with round bottomUnique 1D barcode and human readable charactersManufactured from polypropylene to withstand temperatures to -196°CCaps designed for a secure seal and compatible with automatic decapping equipmentLocking baseSterile SAL 10⁻⁶Certified nonpyrogenic and DNase/RNase-free