VENTANA PD-L1 (SP142) Assay (CE IVD)
VENTANA PD-L1 predictive assays identify patients who are most likely to respond to specific therapies, generating results you can trust so that you can make timely diagnostic decisions and therapeutic choices. We support your expertise by providing you with the tools that you need to successfully implement these assays into your laboratory and interpret them proficiently.
Great results, I like it very much.
Prognostic marker for triple negative breast cancer and other tumors
Staining of PD-l1 is excellent and easy to use on the Benchmark Ultra.
Review Date: 6 Jun 2020
Reliable results with better patient outcomes!
Clinical laboratory
Using a small breast biopsy specimen, it is possible to clearly determine if atezolizumab is the best treatment.
Review Date: 13 Dec 2019
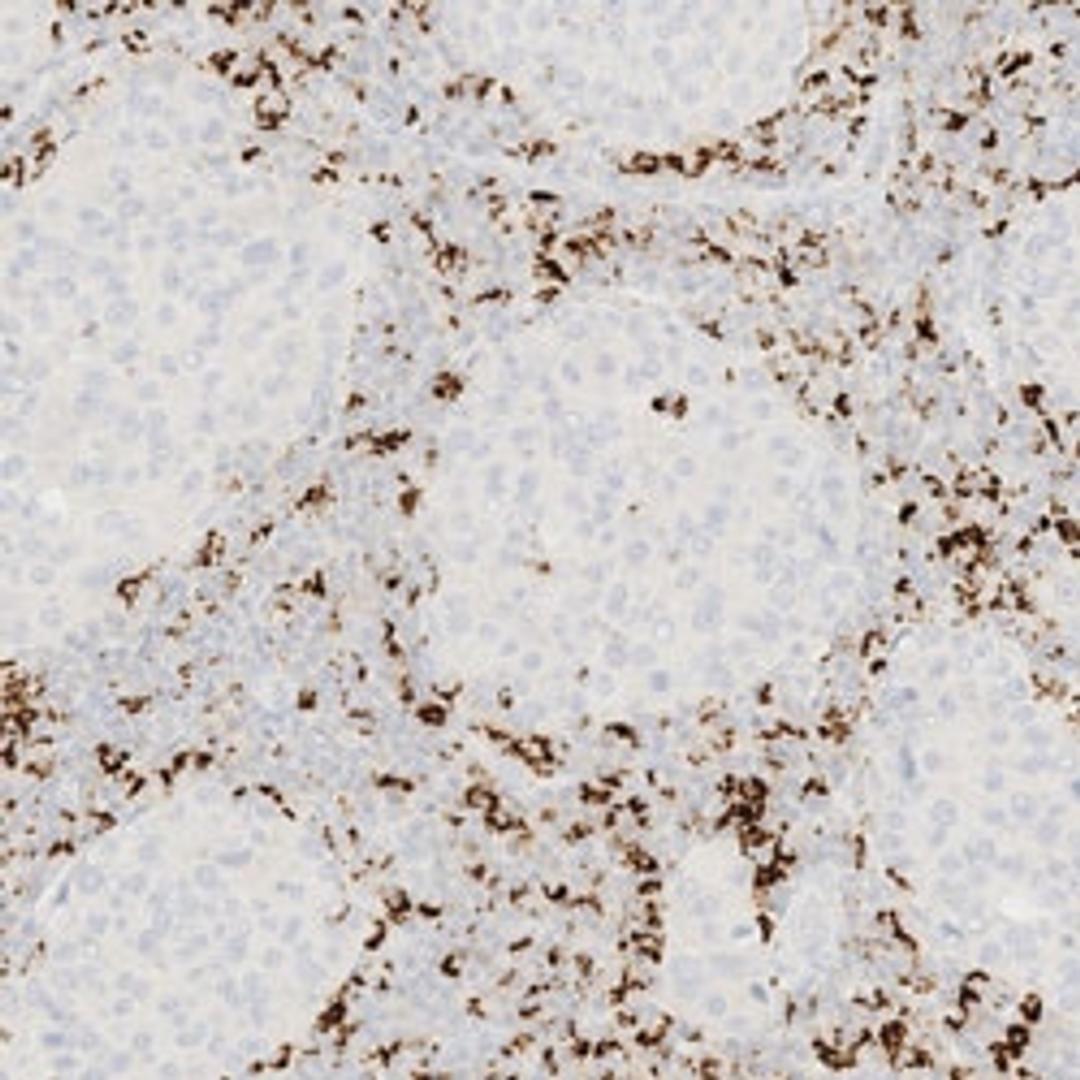
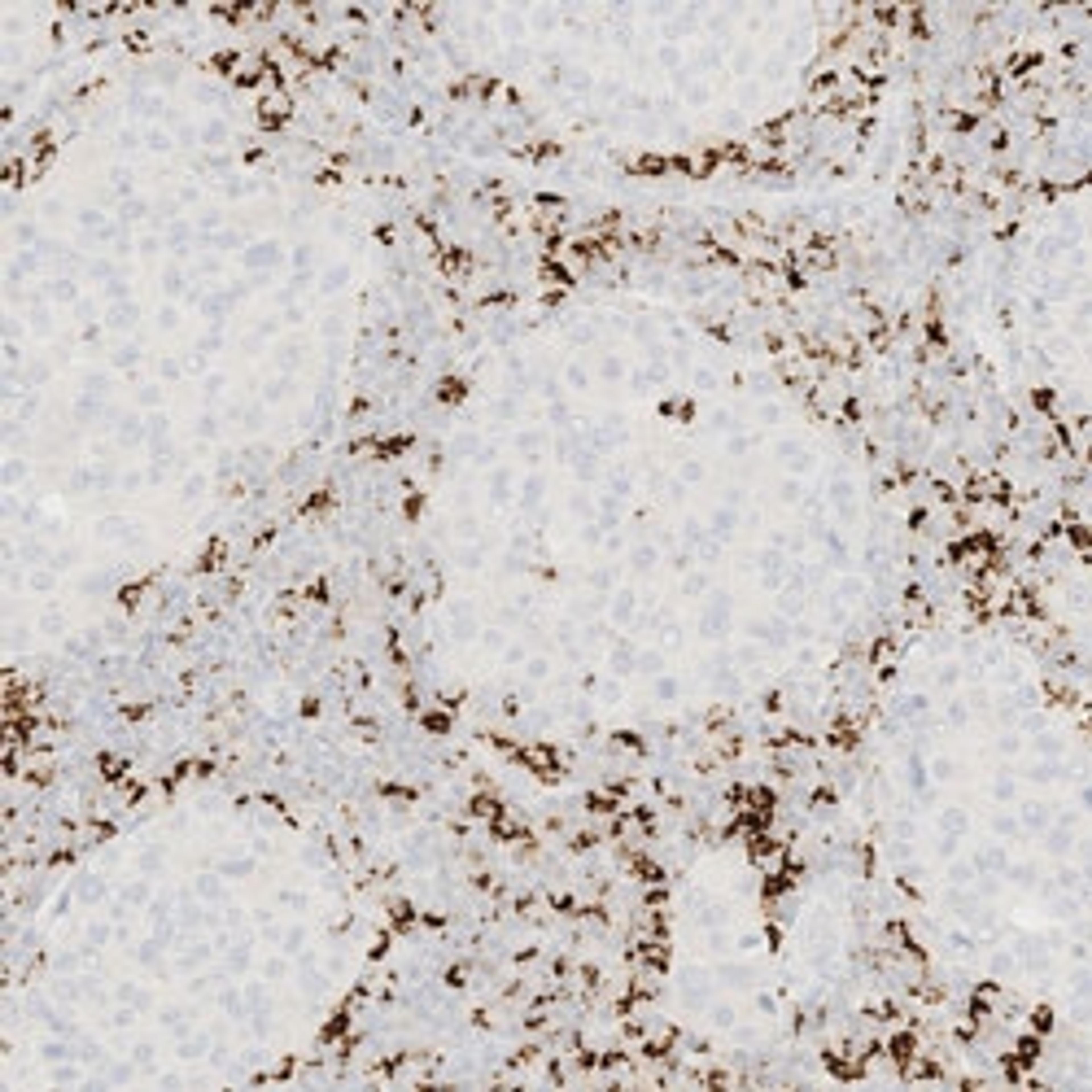
VENTANA PD-L1 (SP142) Assay is intended for the immunohistochemical assessment of the programmed death-ligand 1 (PD-L1) protein in tumor cells and tumor-infiltrating immune cells in formalin-fixed, paraffin-embedded (FFPE) tissues indicated below stained with OptiView DAB IHC Detection Kit and OptiView Amplification Kit on a BenchMark IHC/ISH instrument.
Determination of PD-L1 status is indication-specific and evaluation is based on either the proportion of tumor area occupied by PD-L1 expressing tumor-infiltrating immune cells (% IC) of any intensity or the percentage of PD-L1 expressing tumor cells (% TC) of any intensity.
VENTANA PD-L1 (SP142) Assay is indicated as an aid for identifying patients for treatment with the therapies listed in Table 1 for the respective indications and cutoffs in accordance with the approved therapeutic product labeling.
VENTANA PD-L1 (SP142) Assay may be associated with enhanced patient benefit with the therapies listed in Table 2 for the corresponding indication and cutoffs in accordance with the approved therapeutic product labeling.