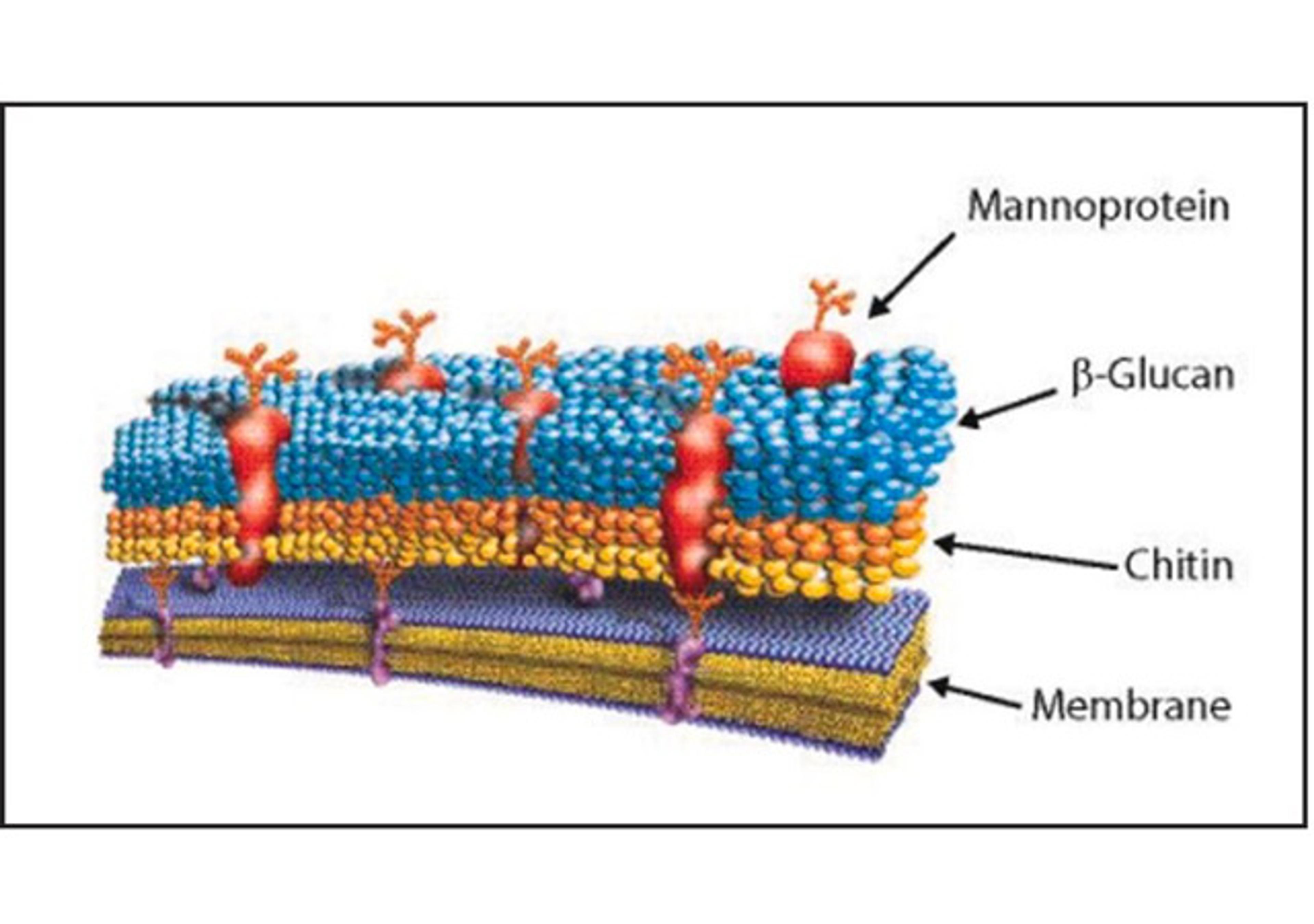
Products & ReviewClinical Diagnostics
SARS-CoV-2 & RSV
LumiraDxAvailable: Worldwide
Is it RSV? Is it COVID-19? Actionable results in minutes. Verify potential infection and help guide infection control measures quickly with a rapid microfluidic assay that provides actionable results in 12 minutes, for patients suspected of RSV and/or COVID-19.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Test benefits
- Microfluidic immunofluorescence assay
- Optimize patient and clinical flow - 1 sample, 2 results, 12 minutes
- Helps guide infection control measures quickly
- Sample Type: nasal swab
- Prepared sample can also be used with LumiraDx SARS-Cov-2 & Flu A/B Test
- Easy to implement at point of care
- Time to result in as quick as 12 minutes
- No refrigeration storage required: room temperature storage (2-30°C)
- Minimal handling steps
- Small and portable Instrument