Products & ReviewClinical Diagnostics
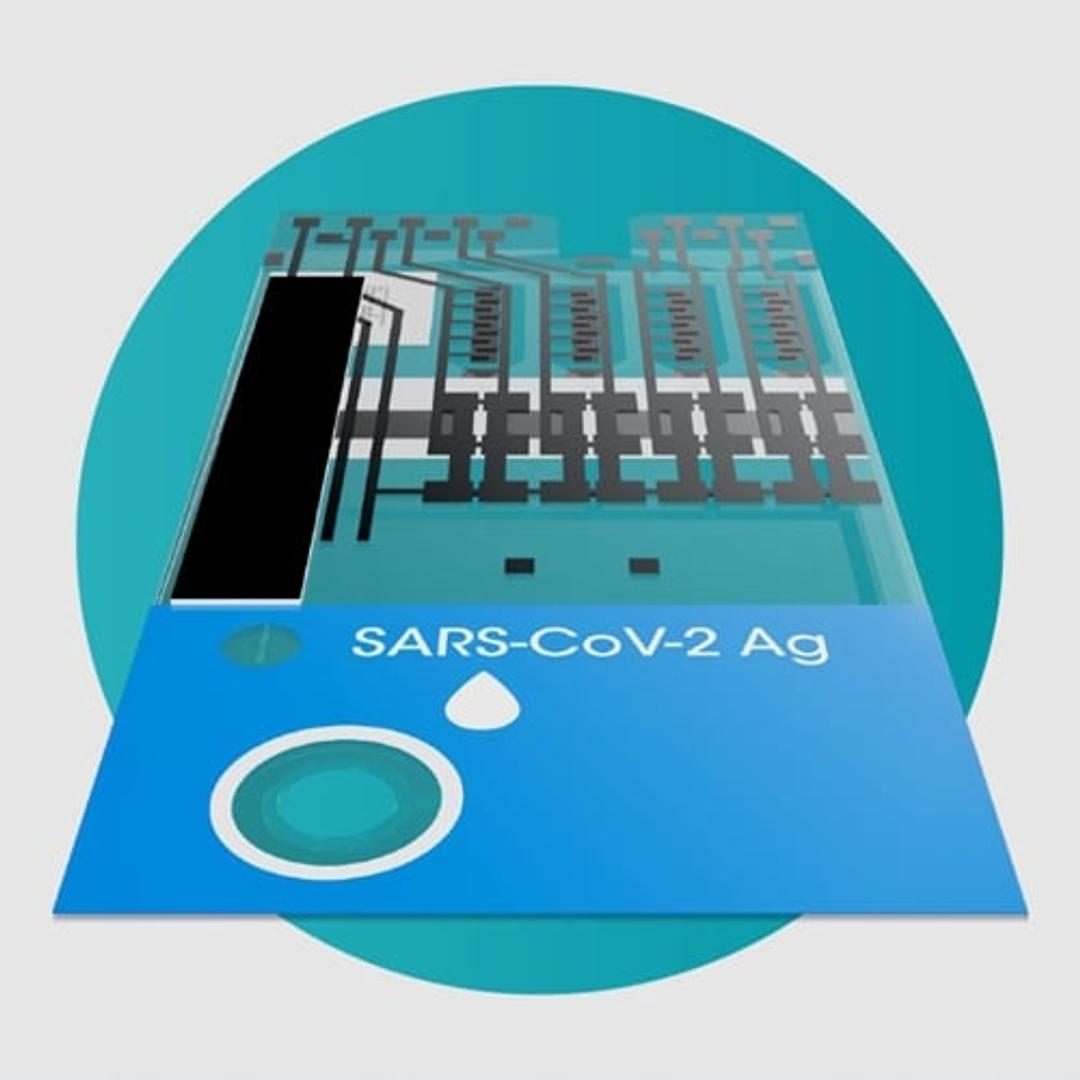
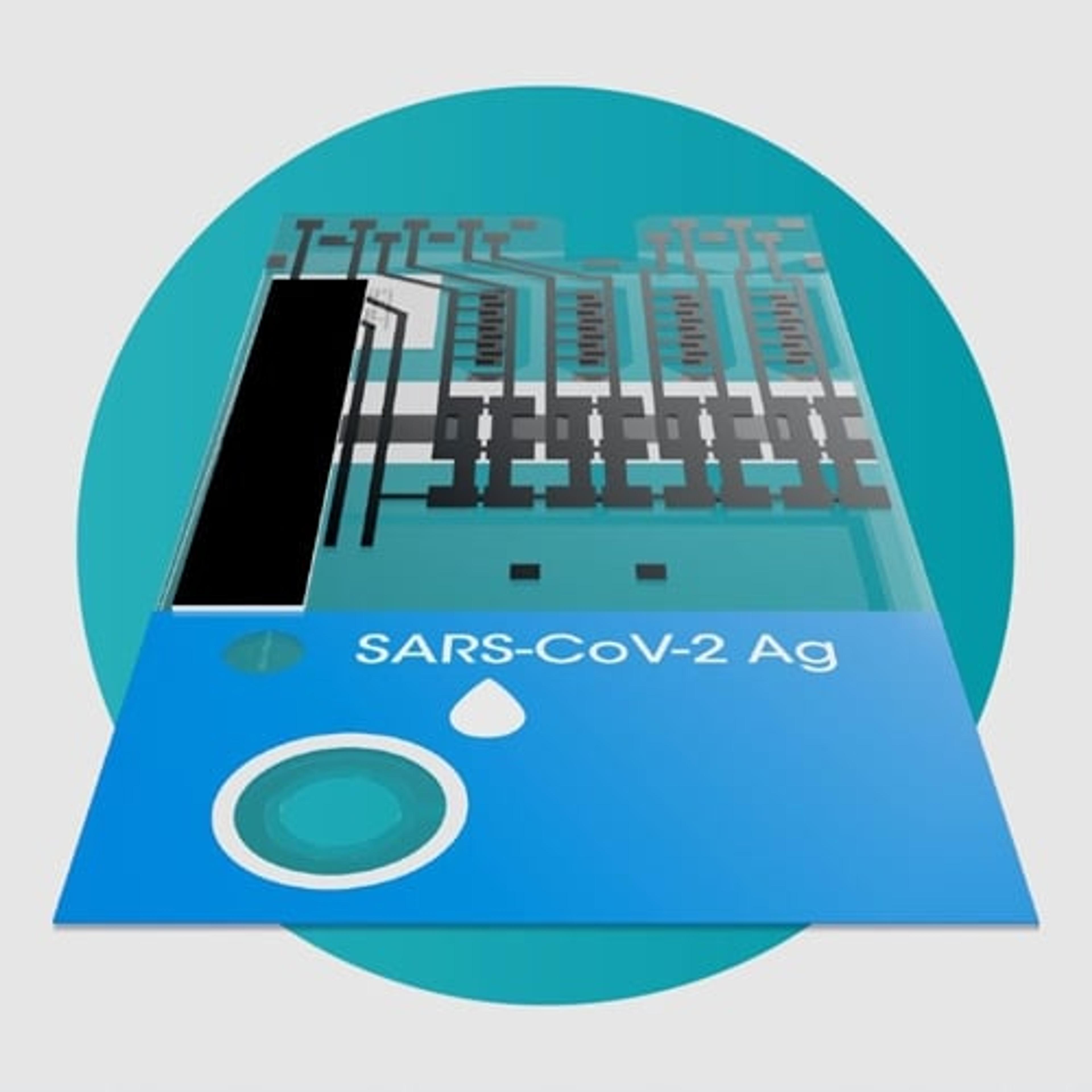
SARS-CoV-2 Ag Test
The LumiraDx SARS-CoV-2 Ag Test is a microfluidic immunofluorescence assay for the direct and qualitative detection of nucleocapsid protein antigen in nasal and nasopharyngeal swab specimens from individuals suspected of COVID-19 or asymptomatic individuals. Used with the LumiraDx Platform the test delivers rapid results at the point of care.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Test benefits
The use of a LumiraDx SARS-CoV-2 Ag Test on the LumiraDx Instruments will enable the physician to verify infection quickly, begin proper treatment and to initiate isolation precautions helping prevent further spread of infection.
- Easy to implement in point of care settings
- Clinical performance
- 7.6% positive percent agreement
- 96.6% negative percent agreement
- Analytical performance with a limit of detection of 32 TCID50/mL
- RT-PCR comparable results within 12 days of onset of symptoms