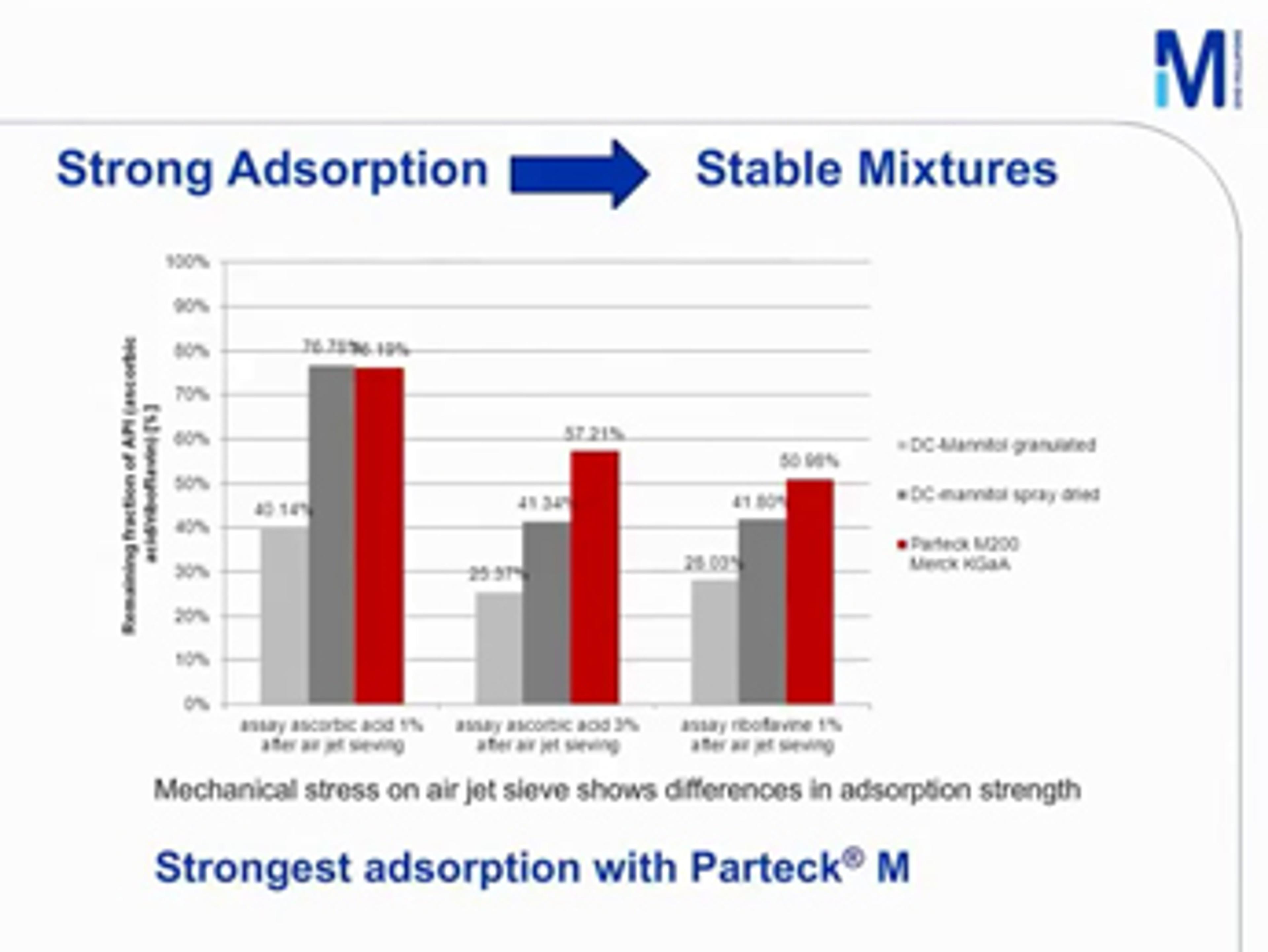
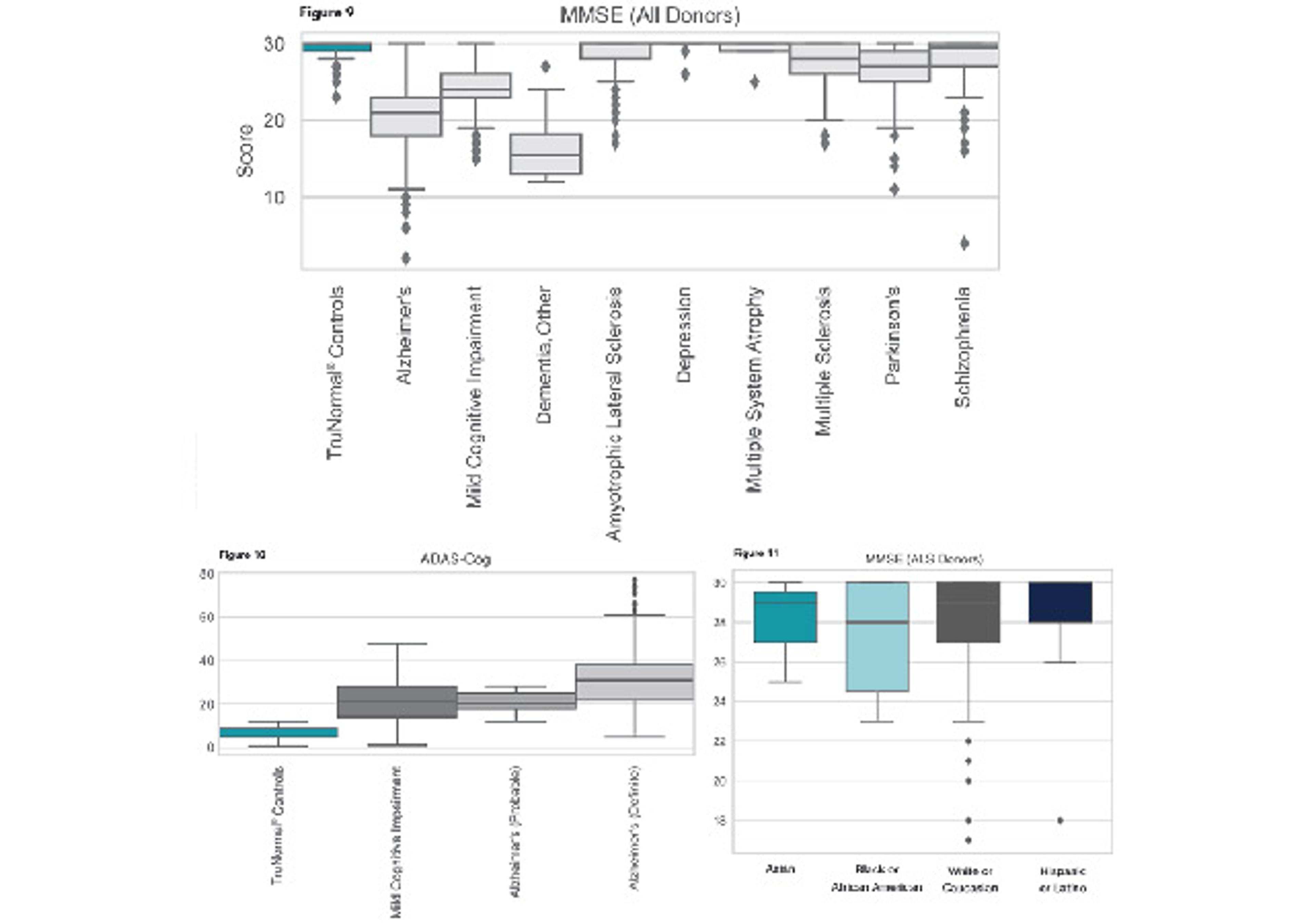
Parteck® M
Advanced technology for excellent tabletting properties Mannitol is a sugar alcohol that is especially suitable as an excipient for advanced, highly active APIs due to its non-hygroscopicity and low content of reducing sugars. Merck Millipore's outstanding, directly compressible mannitol Parteck® M is your best choice – especially if your tableting aims at rapid release of high-dose or low-dose actives.Parteck® M belongs to t…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Advanced technology for excellent tabletting properties
Mannitol is a sugar alcohol that is especially suitable as an excipient for advanced, highly active APIs due to its non-hygroscopicity and low content of reducing sugars. Merck Millipore's outstanding, directly compressible mannitol Parteck® M is your best choice – especially if your tableting aims at rapid release of high-dose or low-dose actives.
Parteck® M belongs to the Parteck® product family of excipients with unique particle properties developed under the Functional Particle Engineering concept. This concept comprises a number of proprietary technologies which allows specialty excipients with outstanding functionalities especially for the design of solid dosage forms.
The extraordinary properties of Parteck® M mannitol allow you to press robust, high-quality tablets directly without the need for additives. This way you achieve compact, more concentrated tablets with fewer process steps and only low compression forces – thereby minimizing wear on your tableting equipment.
Parteck® M Benefits:
- Directly compressible mannitol
- High compactibility at low compression forces
- Rapid disintegration
- High dilution potential
- EMPROVE® documentation