MenoCheck picoAMH ELISA
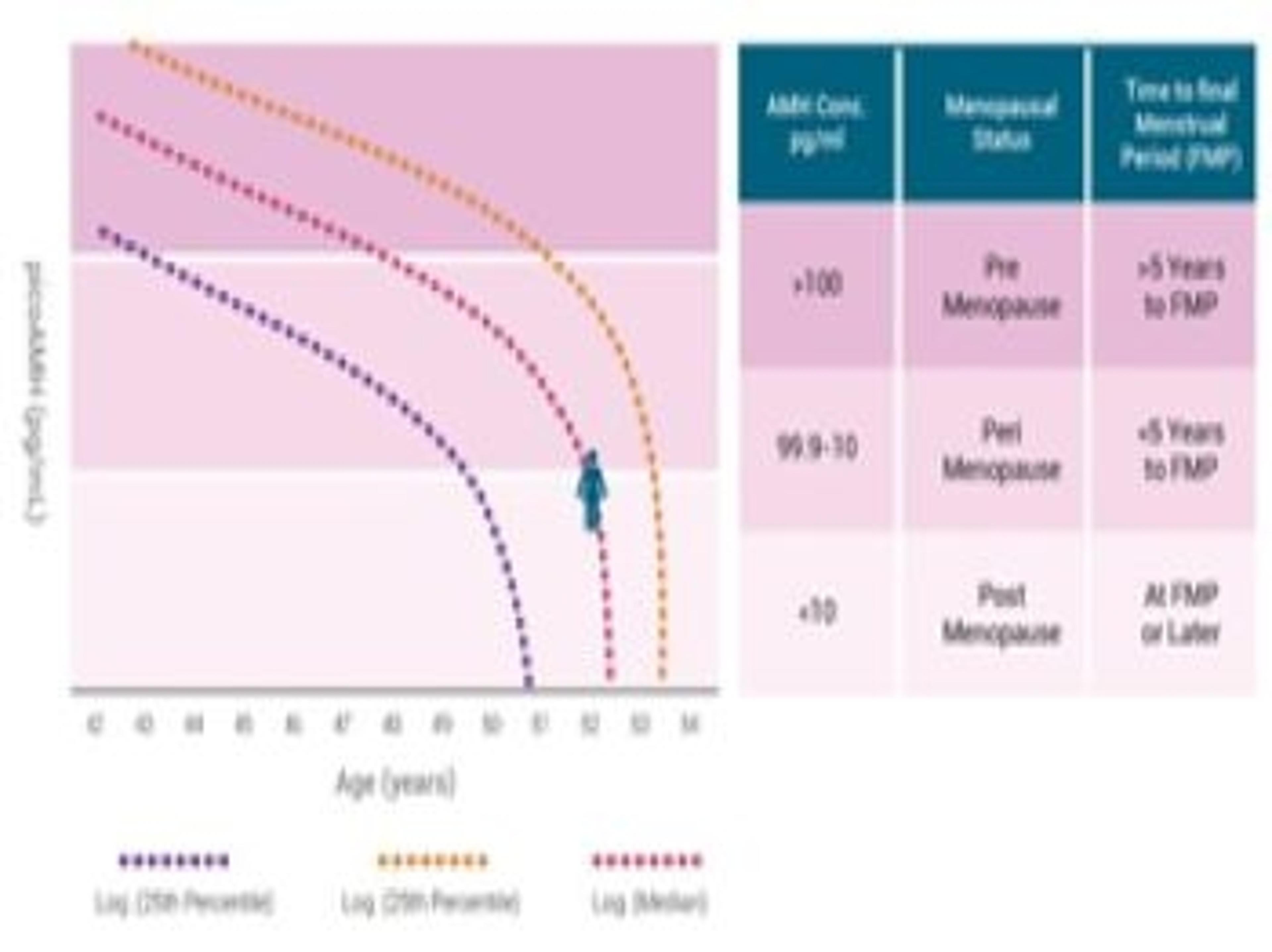
The MenoCheck® picoAMH ELISA is FDA cleared for in vitro diagnostic use as an aid in the determination of menopausal status in women between 42 and 62 years of age. This is the only AMH test with the sensitivity to quantify declining AMH concentrations in women who are entering their menopausal transition.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Great kit.
Translational research
Very effective in detection at picogram level. Excellent.
Review Date: 1 Aug 2019 | Ansh Labs
Nice kit.
Testing menupause
It was easy to use. Price was reasonable.
Review Date: 26 Jun 2019 | Ansh Labs
The Ansh Labs' MenoCheck picoAMH ELISA is a quantitative three-step sandwich immunoassay and is perfectly suited to the measurement of declining levels of AMH. The Ab pair was developed against specific linear epitopes on the associated dimers of AMH thus ensuring specificity and consistency of AMH detection, no detectable cross-reactivity to other isoforms of AMH, different conformations of AMH, or other TGF-Beta superfamily hormones.
- Calibrators are rHum AMH
- xhibits no interference by complement or heterophilic antibodies.
- Analytical measurable range of 7.6 – 1,091 pg/mL.
- Limit of quantitation is 3.2 pg/mL.
- Total 4.5 hour incubation at room temperature.
Ansh Labs, located just south of Houston Texas, is an FDA registered manufacturer of diagnostic test kits, antibodies, antigens, and proteins.