B.I.QUANT-PS 2.0 (For research only)
B.I.QUANT-PS 2.0™ Bruker IVDr Quantification in Plasma/Serum (for research use only)

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
The Bruker IVDr platform is optimized towards supporting the analysis of epidemiological studies with large scale, dedicated clinical and translational trials, and high throughput Biobank quality control. Based on nuclear magnetic resonance (NMR) spectroscopy, the standardized Bruker IVDr Methods module (B.I.Methods) guarantees high reproducibility and transferability under full automation at anytime and anywhere what is not achievable by any other analytical tool.
This standardized platform allows to combine different solutions. For plasma/serum, it means Bruker IVDr Lipoprotein Subclass Analysis (B.I.LISA™) and automated quantification of small molecule metabolites (B.I.QUANT-PS™) using the same experiment. Now we upgrade the quantification from 26 metabolites up to 41 metabolites in B.I.QUANT-PS 2.0.
Key Benefits:
- Ease of use, simple and rapid sample preparation
- Fully automated quantification of 41 metabolites
- Different analytes classes can be quantified simultaneously in a single run
- Absolute concentration for each metabolite is given due to the calibration with one Quantification Reference sample(B.I.Methods™)
- Validation of all LODs has been done following ISO 17025 guidelines for wet spiking
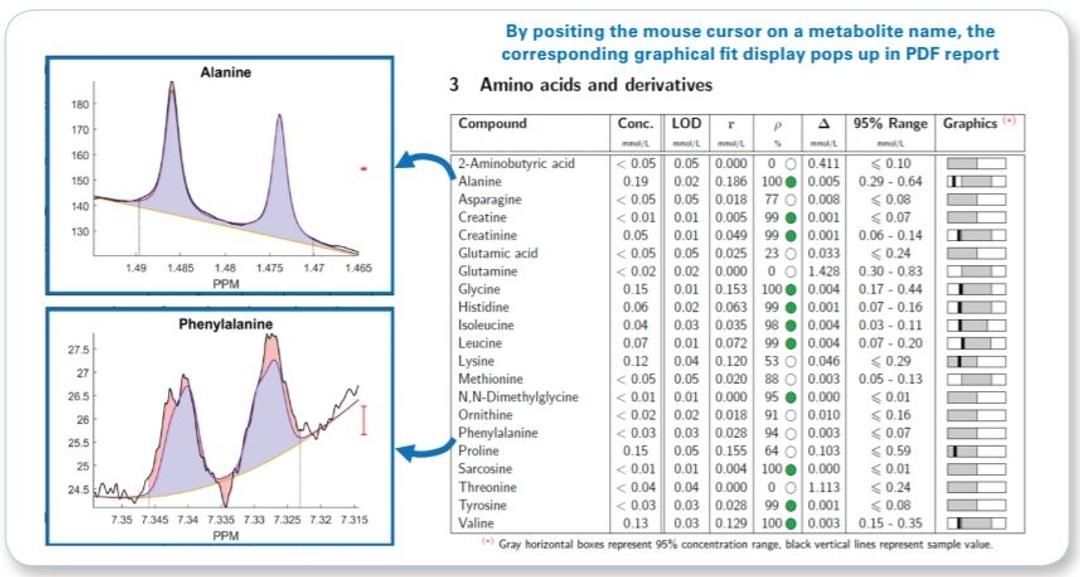
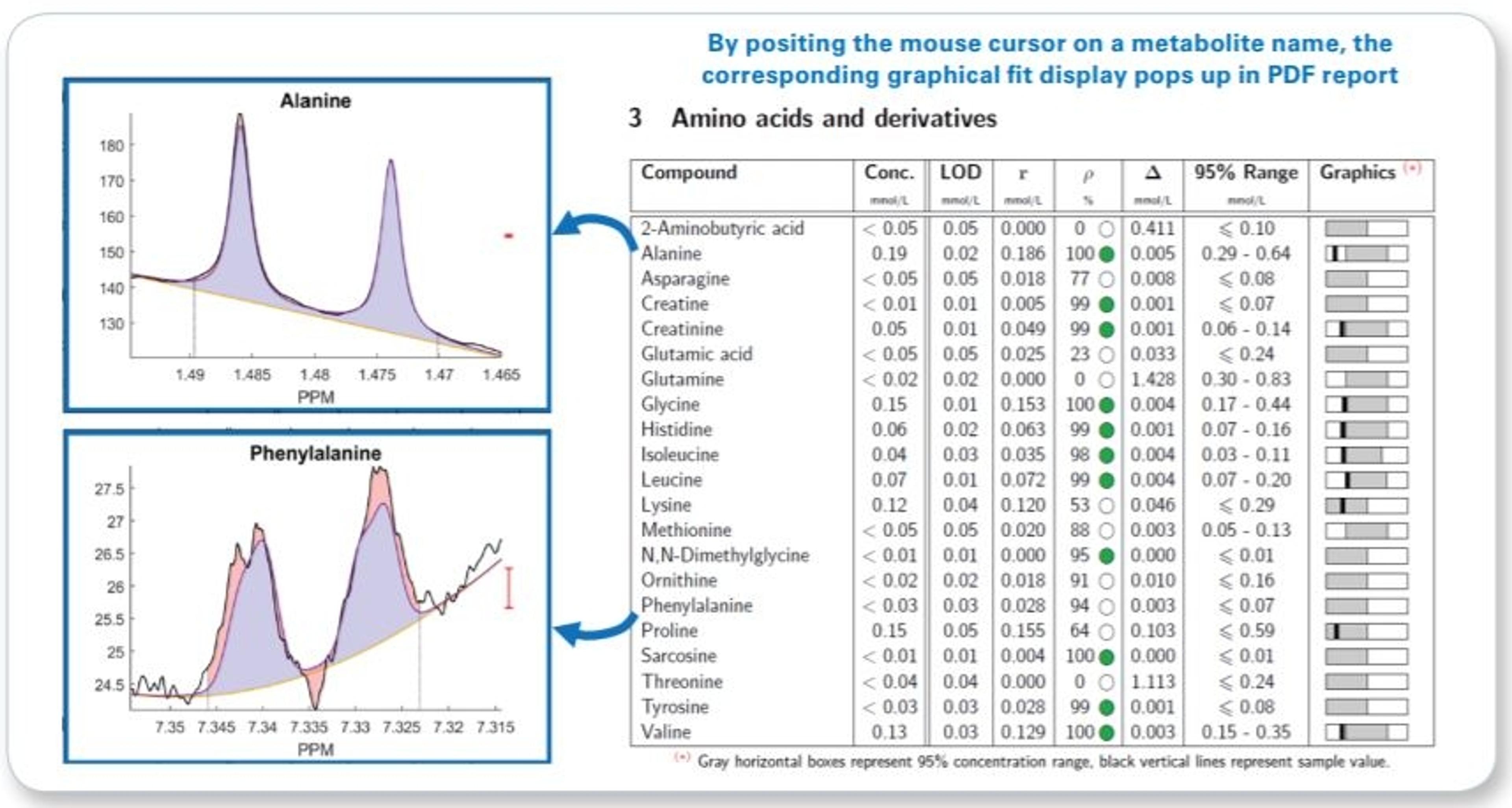
- Raw concentration and additional quantification result assessment information (correlation and residue) for each metabolite is given even below LOD
- Interactive PDF report allowing visual fit ambiguity assessment
- Rapid analysis: daily 120 samples can be prepared, measured and analyzed
- Retrospective analysis possible if the sample was prepared and measured using B.I.Methods module
- Works on plasma or serum sample
Requirements for B.I.QUANT-PS 2.0:
- Bruker IVDr platform or compatible AVANCE™ III HD or AVANCE NEO 600 MHz systems
- Use of B.I.Methods module and SOPs for plasma/serum for sample preparation and measurement
- Regular quality control of the absolute temperature, solvent suppression and quantification reference sample (preferably daily using automated quality control tool including in B.I.Methods)
- Access to Bruker Data Analysis server for fully automated remote analysis (transfer of spectra after measurement to Bruker server via private ftp, back-transfer of result PDF report and XML file)