CyTOF™ XT PRO
The widest immune coverage, at faster speeds. Identify novel disease drivers and design better therapies.
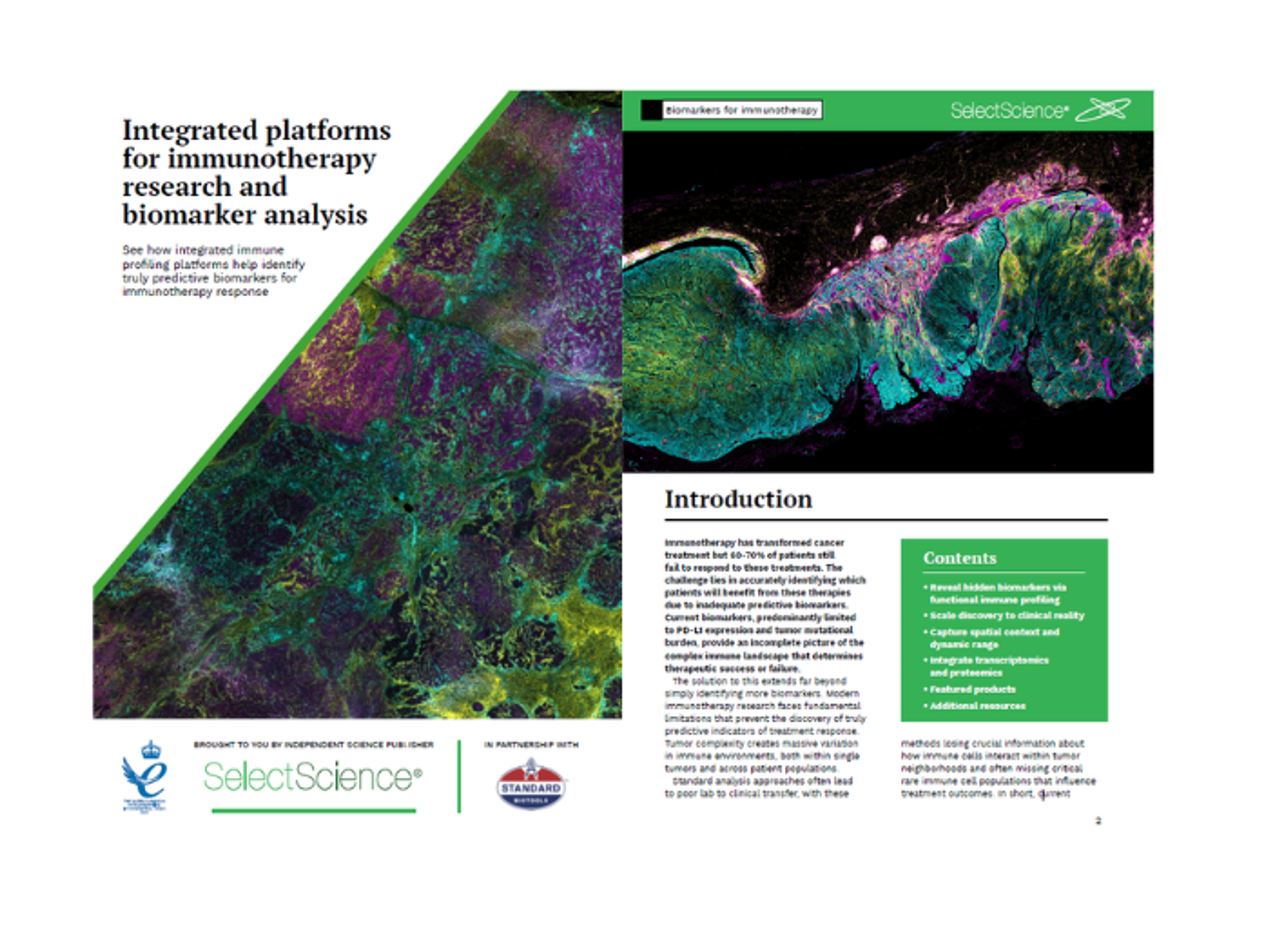
Analyzing immune responses at a scale not possible with other methods, the CyTOF XT PRO system facilitates large immunophenotyping initiatives while meeting regulatory needs for GLP labs. This advanced system brings ease and accuracy to large-scale clinical trials by combining proven reproducibility with enhanced throughput capabilities and 21 CFR Part 11 compliance-enabling software.
The CyTOF XT PRO system takes proven mass cytometry to speeds of up to 4x faster, improves walk-away automation, and advances sample barcoding capabilities to standardize and streamline analysis for larger studies. This powerful cytometry platform with proven sensitivity, high dynamic range and the resolution to capture 50-plus targets simultaneously, including cell surface markers, signaling proteins and cytokines, supports biomarker discovery, patient stratification and treatment response monitoring.
XTra Speed
Combines up to 4x enhanced throughput with automated sample handling to expedite insights.
XTra Reproducibility
Demonstrates consistency across sample collection methods and a <9.2 %CV regardless of workflow, enabling researchers to leverage large cohorts, longitudinal sampling, and multi-site collections with ease and accuracy.
XTra Compliance
Ensures secure and audit-ready data management with integrated 21 CFR Part 11 compliance-enabled software to meet relevant requirements for clinical research.
XTra Scale
Advances barcoding capabilities – a powerful method for harmonizing large datasets of more than 100 samples in a single tube, minimizing technical variability and reducing workflow burdens for clinical trials.
For Research Use Only. Not for use in diagnostic procedures