Cryo-Confocal Imaging with Airyscan
Improving resolution and signal-to-noise in cryo-fluorescence microscopy with ZEISS LSM 8 confocal microscopes and Airyscan
24 Apr 2016

Studying the fine details of biological cells and tissues not only requires a microscope which can attain high resolution images, but also a sample preparation technique that preserves the structures in a close-to-life or near-native state. Rapid-freezing techniques allow to maintain the high water content of cells and immobilize the sample in a near-native state for cryo-imaging by fluorescence, electron or X-ray microscopy. A major limitation for correlative cryo-fluorescence microscopy is the current unavailability of cryo-immersion optics that could yield a higher numerical aperture.
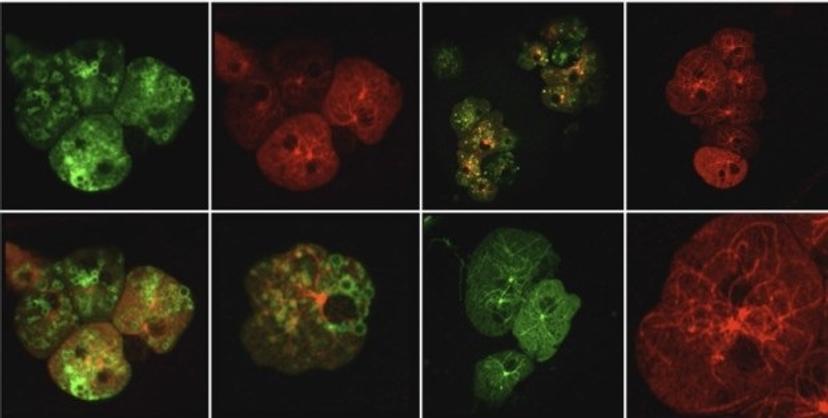
Dictyostelium discoideum cells, cryo-fluorescence imaging. Sample courtesy of Dr. Günther Gerisch, MPI of Biochemistry, Martinsried.
Combining ZEISS LSM 880 with Airyscan and cryo-fluorescent imaging utilizing the Linkam CMS196 cryo-stage allows to obtain a significant increase in resolution and signal-to-noise (SNR) compared to standard confocal images under cryoconditions. ZEISS Airyscan, with its novel confocal detection concept, enables the user to record high-quality cryofluorescence data even without immersion optics.
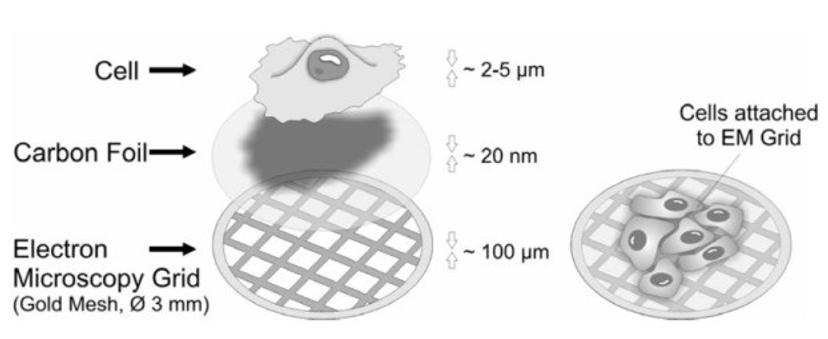
Growing cells on electron microscopy grids. Various kinds of eukaryotic cells can be cultured on gold specimen supports for electron microscopy.
Biological objects such as cells are primarily composed of carbon compounds and up to 70 percent water. Studying the fine details of cells and tissues in their native aqueous environment not only requires a microscope which can attain high resolution images, but also a sample preparation technique that rules out the possibility of any structural changes. Vitrification is a cryo-preparation technique which allows to maintain the high water content of cells, by freezing them to a temperature of app. -190° C within a matter of milliseconds. This fast-freezing process prevents the water molecules from crystallizing and forms a glass-like layer of amorphous ice that preserves cellular structures in a near-native state.
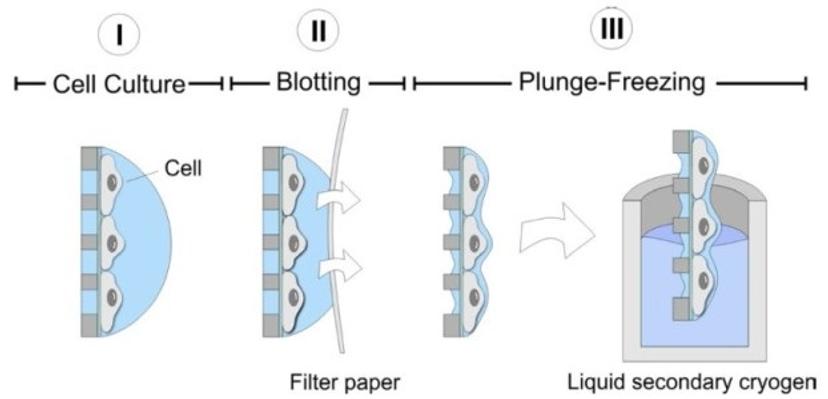
Fast-freezing of cells on EM grids by plunge-freezing into a secondary cryogen (e.g. ethane). The rapid freezing process vitrifies the cells, preserving them in amorphous ice.
Prior to vitrification, cells are cultivated on electron microscopy (EM) grids coated with thin carbon films. The grids are made out of a non-toxic material (typically gold). Immediately before freezing, the residual growth medium or buffer is removed by blotting with a filter paper, leaving behind a thin liquid film covering the cells on the EM grid. The EM grid is then rapidly immersed into a secondary cryogen (e.g. ethane or propane), leading to vitrification of the cells. Once frozen, the sample needs to remain at liquid nitrogen temperature at all times.
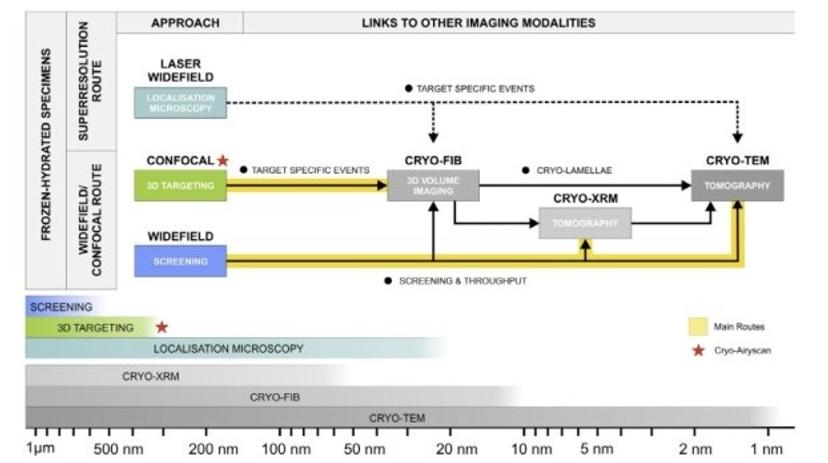
Correlative routes in cryo-fluorescence microscopy.
Cryo-fluorescence imaging is a relatively new discipline, which combines optimal sample preservation by cryo-preparation methods with the advantages of fluorescence labelling of biological structures. It is becoming more and more popular in correlative microscopy, where fluorescence imaging is used to identify areas or events of interest within cells, which are then examined in detail by electron microscopy. A major advantage of correlative cryo-imaging workflows over resin embedding workflows is that no compromise between ultrastructural or fluorophore preservation is made. Cryo-conditions offer superior structural preservation and enhanced photostability during imaging.
This free ZEISS Application Note demonstrates, for the first time, cryo-confocal Airyscan imaging of vitrified specimens. The data proves that under cryo-conditions, even without immersion optics, a significant increase in resolution and SNR can be obtained with Airyscan compared to standard confocal imaging

