Streamline food safety analysis through automation
Exploring food safety monitoring, USDA expert, Steve Lehotay, advocates for change, and highlights the need for innovation in current practices to enhance routine analysis
13 Jan 2025

Steve Lehotay, lead scientist in the US Dept. of Agriculture (USDA) Agricultural Research Service
Steve Lehotay, a Lead Scientist in the US Dept. of Agriculture (USDA) Agricultural Research Service at the Eastern Regional Research Center near Philadelphia, Pennsylvania, shares his laboratory's proven strategies for boosting productivity and ensuring accuracy in output. Lehotay is a pioneer of the 'quick, easy, cheap, effective, rugged, and safe' (QuEChERS) method for pesticide residue analysis in foods, along with its recent update (QuEChERSER) to cover an even wider analytical scope. His philosophy underscores the importance of embracing cutting-edge technologies to streamline lab operations.
Lehotay explains how his research involves developing better analytical methods to monitor chemical contaminants in foods, with the goal of improving accuracy and efficiency. “We address all aspects of the analytical process, using a variety of tools and techniques to efficiently achieve fit-for-purpose data quality objectives. This involves developing and validating a ‘mega-method’ capable of accurately identifying and quantifying as many analytes of concern as possible in any food matrix.”
In his role, Lehotay is dedicated to providing effective and efficient methods for global implementation in routine monitoring programs. This includes meeting the needs of the USDA Food Safety and Inspection Service for their National Residue Program, which covers meat, poultry, processed eggs, ready-to-eat meats, and catfish. His research, spanning various chemical analytes in diverse foods, is also applicable in other fields of analytical chemistry, such as forensics, clinical, and environmental analysis.
Fortunately, modern mass spectrometry (MS) techniques, coupled with gas and liquid chromatography (GC and LC), enable ‘quant-identification’ of a variety of small molecule analytes at extremely low detection limits.
Steve Lehotay, Lead Scientist at the Eastern Regional Research Center
Expanding on significant analytical hurdles within his field, Lehotay highlights the immense difficulty of conducting multiclass, multiresidue analyses on potentially thousands of substances, each potentially present at ultra-trace concentrations within complex food matrices. “Fortunately, modern mass spectrometry (MS) techniques, coupled with gas and liquid chromatography (GC and LC), enable ‘quant-identification’ of a variety of small molecule analytes at extremely low detection limits,” he adds.
Lehotay’s team employs GC and LC instruments coupled with either triple quadrupole tandem MS for targeted analytes, or quadrupole/high-resolution (HR) MS to cover nontargeted analytes. “As the scope of analytes and sample types expands, the degree of selectivity in sample preparation must correspondingly expand to accommodate a wider array of physiochemical properties. Thus, my approach is to tailor conditions to minimize co-extraction of typical food components, such as sugars, proteins, cholesterol, fatty acids and other lipids, while still recovering a broad set of regulated contaminants.”
Enhance efficiency and accuracy in routine monitoring
Lehotay believes in approaching the analytical process holistically, emphasizing the integration of every step, from sample processing to reporting, into a streamlined protocol. He emphasizes that achieving acceptable quality results in the most cost-effective, convenient, and timely manner is a priority for all food safety laboratories. Embodying this commitment, during the 2000s, Lehotay and his partners pioneered the development of the 'quick, easy, cheap, effective, rugged, and safe' (QuEChERS) approach for analyzing pesticide residues in foods. Recently, they introduced the QuEChERSER concept which expanded the analytical scope to include veterinary drugs, as well as environmental and other contaminants in fatty and non-fatty foods. QuEChERSER also incorporates quality control (QC) at every stage, introducing unique QC standards before each step in the procedure, and evaluating recoveries to demonstrate method performance. This facilitates troubleshooting if needed to improve overall precision.
Minimizing instrument downtime is also critical to achieving both high-throughput and optimal performance in monitoring labs.
Steve Lehotay, Lead Scientist at the Eastern Regional Research Center
“For efficient routine monitoring, it is essential that highly rugged methods are used, as instrument downtime can be very costly,” says Lehotay. “Minimizing instrument downtime is also critical to achieving both high-throughput and optimal performance in monitoring labs.” The key to minimizing instrument downtime, he reveals, is successfully meeting the challenge to maintain system suitability. “Even low levels of fats in final extracts can damage chromatographic columns and MS instruments, and nearly all foods have some degree of lipid components that must be removed,” adds Lehotay. To address this challenge and uphold system cleanliness, Lehotay's team employs high capacity, thick-film megabore columns in low-pressure fast-GC, along with dual alternating column backflushing in LC, to manage the complex food extracts effectively. His lab also utilizes precipitation and solvent-exchange in LC to avoid lipid co-extractives. Additionally, the team’s chromatograms are faster than the norm, at ~10 min long for both GC and LC, which helps increase sample throughput.
Embrace automation
Soon after joining the USDA in 1992, I recognized three enduring trends in real-world analytical chemistry: streamlining, miniaturization, and automation.
Steve Lehotay, Lead Scientist at the Eastern Regional Research Center
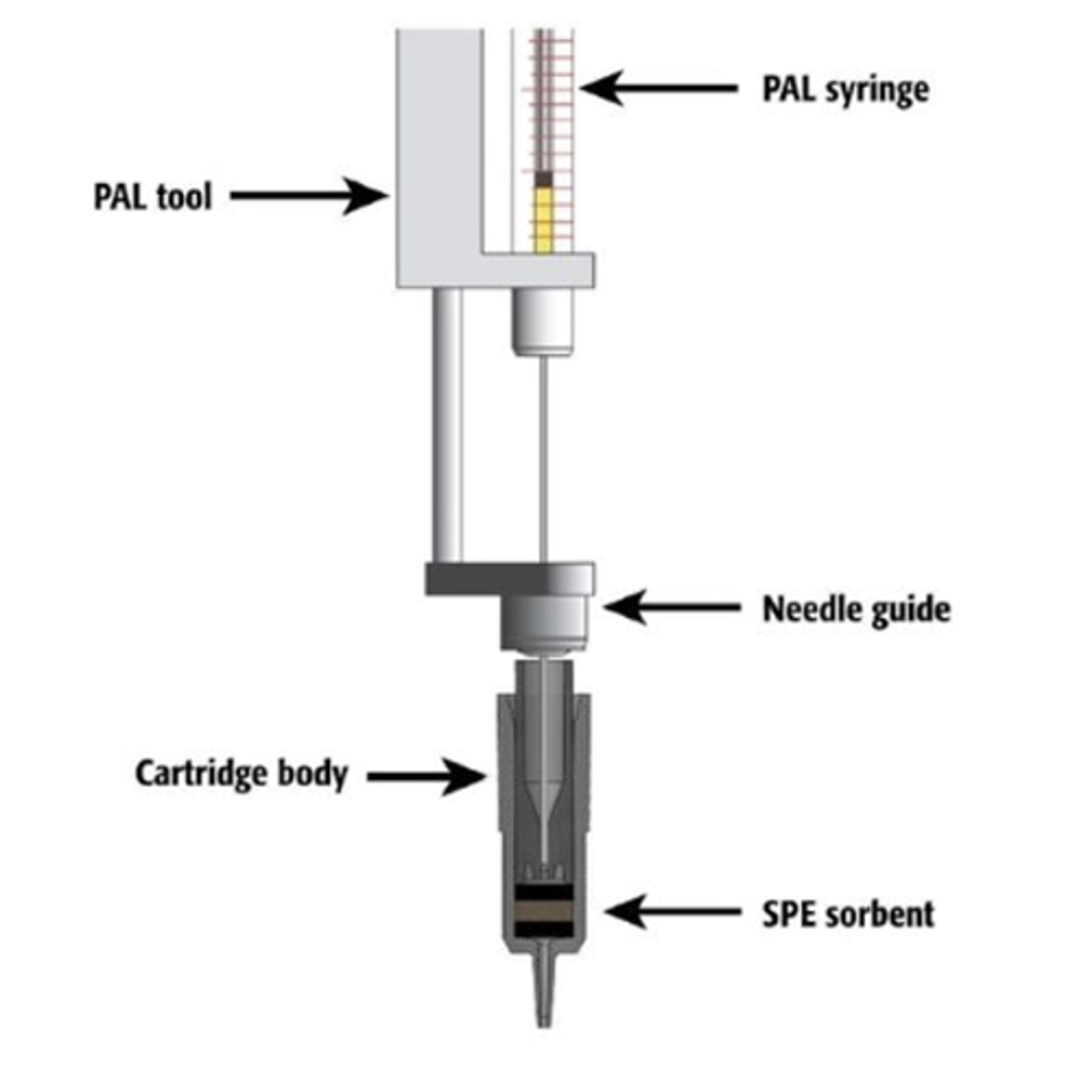
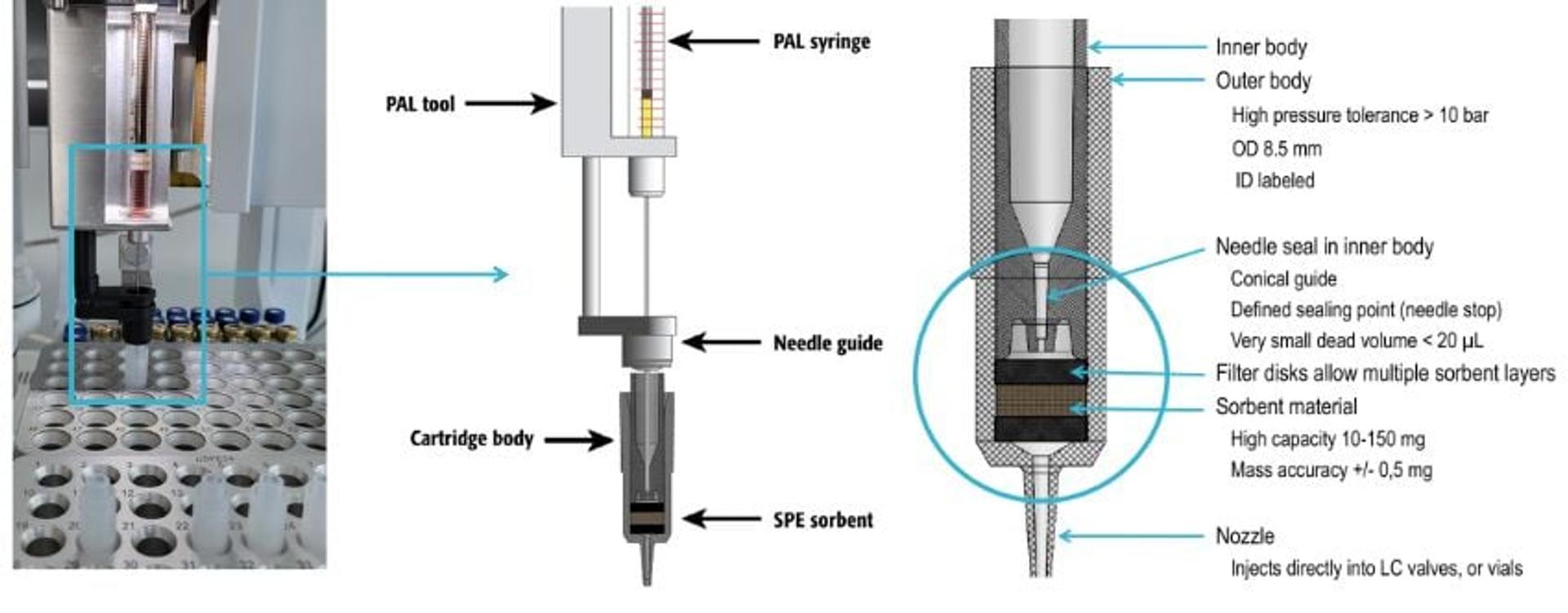
To further ensure system quality, Lehotay encourages the widespread adoption of automated methods, citing improved cleanup, precision, and sample throughput. “Examples of applying automation include ‘instrument-top sample preparation’ (ITSP) and miniaturized solid-phase extraction (µ-SPE),” he explains. “In our lab, we commonly use this for GC, where the robotic autosampler performs miniaturized SPE cleanup of extracts just before each injection. The commercialized mini-cartridges for GC typically contain sorbent combinations of anhydrous magnesium sulfate, primary secondary amine, and octadecylsilane for effective dehydration and adsorption of fatty acids and other lipids in the extracts.” Although the concept of mini-cartridge SPE and sorbent combinations predates their utilization in his laboratory, Lehotay and his team pioneered the integration of automation into this approach, as described in their open-access paper in 2016. Subsequently, they updated QuEChERSER in 2022, incorporating a newly introduced µ-SPE mini-cartridge design1.
Look to the future, while learning from the past
"Performance-based methods require internal validation and participation in proficiency testing programs to help ensure quality of results, and this concept continues to rise in prominence today,” says Lehotay, expressing a similar hope for the widespread implementation of (semi-) automated methods such as QuEChERSER which offers easy incorporation into a turn-key protocol tailored for a variety of instruments across laboratories.
In response to the common complaint that method validation can be time-consuming and laborious, Lehotay advocates for the validation process to be streamlined, drawing parallels to standardization in routine analysis. “Standardized automated methods have been successfully implemented for several decades in routine monitoring applications such as blood and urine testing. Hence, there are no real conceptual or technical barriers to their implementation in food safety labs. However, there are currently few external pressures in the field to alter the status quo, because analyte scope, food testing rates, lab resources, competition, and peer pressure hasn’t changed much in several years,” he explains.
Lehotay notes that in the past, rapid adoption of the QuEChERS method was driven not only by its efficacy but also by regulatory and customer demands for broader pesticide analysis at lower concentrations. “Despite the current status quo, those labs that make the improvements on their own now will gain the benefits sooner and survive, or even thrive, when external forces for change do occur, which is an inevitability. My hope is that labs will proactively embrace efficiency changes such as the ones that come with automation, rather than reacting when compelled to do so.”
To benefit from automated sample preparation methods, eliminating labor-intensive and error-prone procedures while reducing sample, solvent, and expensive standard usage, contact CTC Analytics. CTC Analytics specializes in automated extraction and sample clean-up workflows.
Disclaimer: Mention of brand or firm name does not constitute an endorsement by the US Department of Agriculture (USDA) above others of a similar nature not mentioned. The opinions expressed in this discussion are the author's own and do not reflect the views of the USDA.
References
Michlig, N., and Lehotay, S.J. Evaluation of a septumless mini-cartridge for automated solid-phase extraction cleanup in gas chromatographic analysis of >250 pesticides and environmental contaminants in fatty and nonfatty foods. J Chromatogr A. 2022 Dec 6:1685:463596. doi: 10.1016/j.chroma.2022.463596.