The integral role of human retinal ganglion cells in eye disease
Discover how researchers are better understanding human eye disease by characterizing the subtype diversity and development of RGCs
1 Aug 2022

The study of human eye development is invaluable for studying many visual conditions, including glaucoma, which is one of the leading causes of human blindness. However, studying the development of the human eye is incredibly difficult due to the limited access to genetically and pharmacologically manipulable tissue.
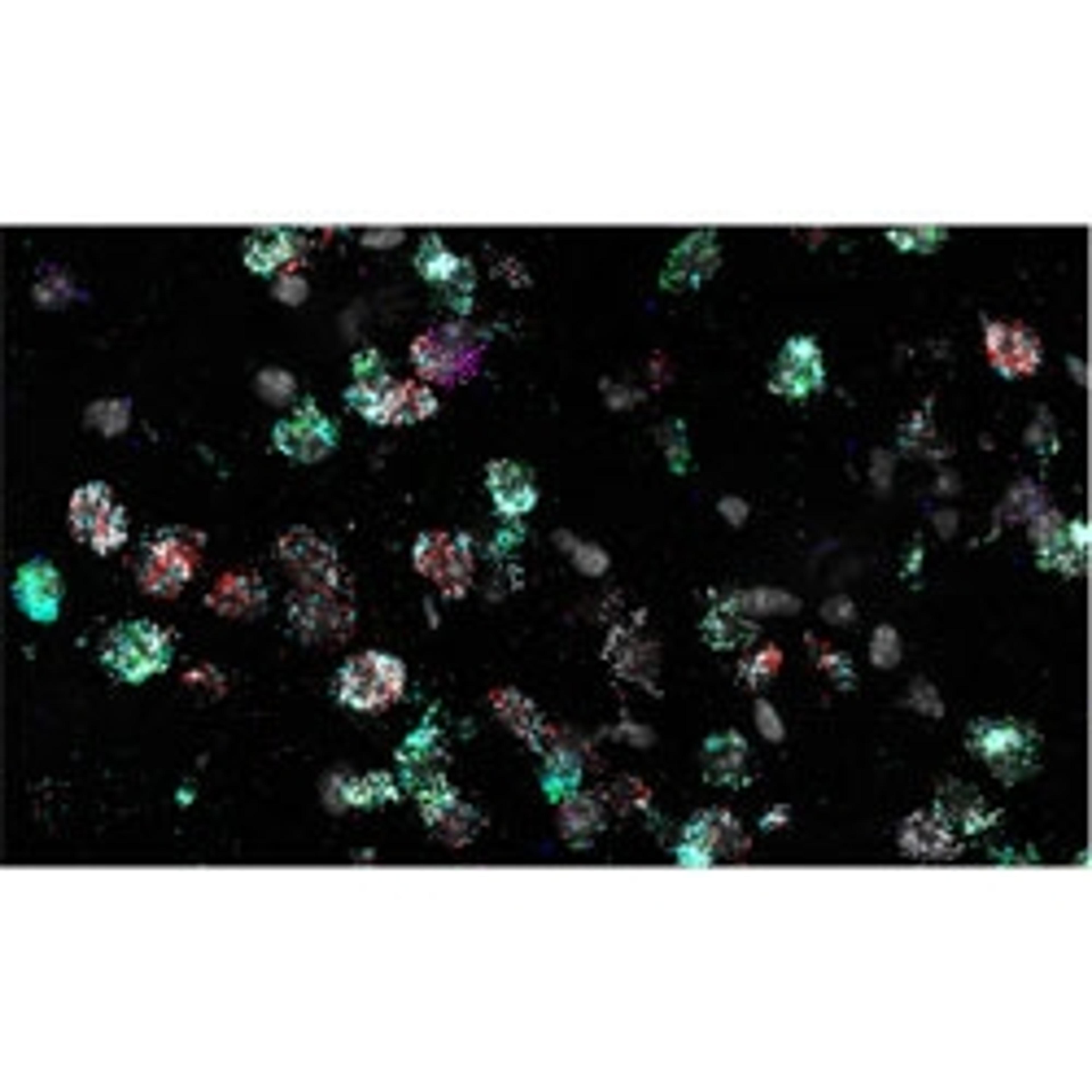
In this article, Brian Guy, a fourth-year Ph.D. student at Johns Hopkins University, explains how he uses human retinal organoids (human stem-cell-derived mini-retinas grown in a dish) to recapitulate the developmental timing and cellular composition of the developing human retina. By combining this organoid technology with HiPlex RNAscope™ ISH technology, researchers are able to interrogate the mechanisms used in human development, and explore what kind of simian molecules or intrinsic factors are controlling the specification of different cells. With this approach, Guy has thus far identified four RGC subtypes in human retinal organoids and will characterize more using HiPlex RNAscope.
Brian Guy recently presented his work in the SelectScience® webinar series: Diversity, development, and dysfunction in the brain. Watch his presentation on demand, here >>
What are the overall goals of your research?
BG: I study retinal ganglion cells (RGCs). These are the projection neurons of the eye, and they send all the visual information from the eye to the brain. RGCs can be divided into many subtypes based on morphology, physiology, projection targets, and gene expression. However, the number of human RGC subtypes is still unknown, and I'm curious as to how many different subtypes there are. While we have a good understanding of these different subtypes and other model systems, our understanding of how many different human RGC subtypes we have, is still very much in its early stages.
What are the key challenges you face when investigating these subtypes?
BG: The biggest challenge is that a lot of these RGC subtypes are exceptionally rare. Some of these subtypes only make up a fraction of a percent of the total RGC population, so trying to find them is like trying to find a needle in a haystack. However, if you have the right genetic markers, the search is a lot easier.
What are the benefits of RNAscope technology?
BG: The HiPlex RNAscope technology lets me scan/screen up to 48 different genes. I've done 12 Plex runs, 36 Plex runs, and these 48 Plex runs allow me to examine many different subtypes in a single experiment. In order for you to get a single RGC subtype, you need to have a minimum of three to four different marker genes. If you want to capture more than one subtype in an experiment, you need to have dozens of different genes at once. That's what this system allows me to do. It allows us to look at these different subtypes in a wide scope in a single experiment.
I think it's incredibly powerful and very accessible. Being able to expand the number of targets that you can visualize into a single experiment is incredibly helpful, plus the reagents are incredibly flexible and you have control over the experiment throughout the entire process, so I highly recommend the system.
There are a lot of user-scale RNA FISH technologies that have become more popular in the last few years, and the HiPlex scope has one powerful advantage that can't be overstated as it enables you to do these experiments without having to use special equipment or having to use a transcriptomics core. You have complete control over these experiments from the beginning to the end. That's something that I appreciate about this system.
What kind of impacts are you hoping to have with this research?
BG: For both glaucoma and macular degeneration, you experience either loss of peripheral vision or loss of central vision outward. Developing a better understanding of what these subtypes are can pave the way for new therapies, or even cell replacement therapies. That is the downstream objective of some of my work, to use stem cell resources as a biological resource for transplantation therapies to restore vision. On top of this, there's a very interesting recent finding that one subtype of RGC impacts your mood. The study showed that mice who don't have this specific subtype experienced symptoms of depression and anxiety. There is a wide degree of variability in all the different processes that these RGC subtypes are working with.
The biggest impact is probably in glaucoma. Glaucoma is one of the most prevalent blinding conditions in the world and it occurs when different RGC subtypes die off as you grow older. There's growing evidence from different model systems that specific RGC subtypes are more susceptible to cell death, but since we don't know what the RGC subtypes are in humans, we don’t yet know what subtypes are dying off in glaucoma. One of the big rationales is if we can better understand the human RGC subtypes, we can better understand what kinds of cells are dying off from glaucoma and develop better therapies for it.
Why is peer-to-peer communication in science important?
BG At the very foundation of sciences is communication. You can't understate its importance. You need to communicate science in order to initiate collaborations and to get advice from colleagues, and you need to convey your findings with webinars, conferences, symposia, lab meetings, and seminars. It's a critical aspect of the scientific method. Science does not exist in a vacuum, there needs to be communication at every level of the scientific process, so it's absolutely essential for any science to work.
Discover more innovative research from neuroscientists leveraging single-cell RNA analysis technologies: