AI microscopy breathes new life into asthma research
Microstructural ‘fingerprints’ of inhalation powders revealed by AI image analysis could help scientists develop better treatments for asthma
30 May 2024
Scientists have demonstrated a new way to investigate the properties of dry inhalation powders that combines correlative X-ray microscopy with AI-powered image analysis. The research opens doors to accelerate the development of generic treatments for asthma and provides insight into how inhalation medicines could be significantly improved.
An estimated 339 million people worldwide have asthma, with approximately 1000 deaths attributed to the condition every day. Many of these deaths could be avoided through the timely administration of respiratory medication, yet access to low-cost therapies is lacking. Research partners Dr. Parmesh Gajjar and Prof. Darragh Murnane view this as a fundamental reason for why a greater number of generic products are needed on the market, but establishing bioequivalence can be challenging. Now, in a study published in the European Journal of Pharmaceutics and Biopharmaceutics, the researchers have demonstrated a unique approach to examining inhaler formulations that could help bridge this gap.
Look at inhalation powders in a new way
Most pharmaceutical ingredients, whether dosed in tablets, aerosols, capsules, or suspensions, are comprised of polycrystalline solids. Understanding the key physicochemical and crystalline properties of such solids is thus essential to ensure that a drug product is both effective and safe. Here, however, inhalation formulations pose a formidable task. At the heart of this challenge is the fact that the very property of the particles that makes them suitable for inhalation – their small size which, at less than 5 µm, is less than the diameter of a human hair – causes them to clump together as agglomerates, making them incredibly difficult to physically characterize.
Consequently, efforts to characterize the structure of dry powder inhalation (DPI) blends have traditionally relied on tests that require destructive sampling of the powder, which are subsequently unable to examine the bulk formulation. These methods include electron microscopy, morphologically directed Raman imaging, dissolution tests, and single-particle aerosol mass spectrometry. Moreover, such techniques have primarily been used to characterize inhalation formulations post-aerosolization, after they have exited the inhalation device.
In contrast, Dr. Gajjar and Prof. Murnane sought to understand the microstructure of formulation blends before aerosolization, which they believed could provide greater insight into how and why the characteristics of formulations lead to inter-patient variability in aerosol characteristics. To this end, the researchers employed X-ray computed tomography (XCT), a non-destructive imaging technique that enables the digital reconstruction of samples in 3D to reveal the size, shape, and arrangement of particles.
“X-ray computed tomography has not really been used in the inhaled space because it presents a really difficult challenge,” explains Dr. Gajjar. “We were stepping into the unknown… trying to push the boundaries.”
Correlative X-ray microscopy reveals drug-rich regions in DPI blends
One such challenge is the fact that the two primary components of DPI blends – carrier particles (typically crystalline lactose) and micronized active pharmaceutical ingredients (API) – differ greatly in size, meaning they require imaging on multiple length scales.
To overcome this, the researchers exploited the unique optics of correlative nano-micro XCT using two XRMs from ZEISS, a model of the Versa series for non-destructive 3D imaging at sub-micron resolution, and a model of the Ultra series for nanoscale 3D imaging.
Images taken using these instruments were processed using two AI-based image analysis tools, PhaseEvolve and DeepRecon, both modules from ZEISS' Advanced Reconstruction Toolbox (ART). PhaseEvolve, a smart algorithm that removes phase fringes, enabled the researchers to better segment different phases. These regions were then correlatively imaged at the nanoscale, using the deep learning tool DeepRecon Pro to reduce noise artifacts and increase the throughput of image processing.
“The AI powered reconstruction DeepRecon Pro cleans up all of the noise behind the images, allowing us to understand the structure of these materials,” explains Dr. Gajjar.
Thus, by combining correlative XCT and AI image processing, the researchers were able to deduce different phases within the powder blends, including air, carrier lactose, and drug-rich regions consisting of agglomerates of API (Fig. 1).
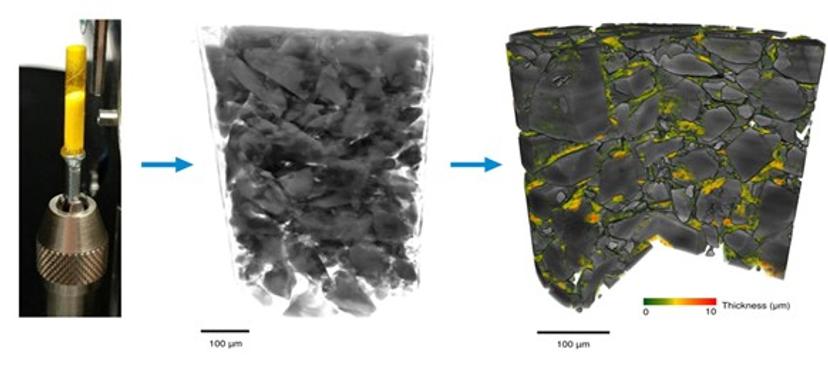
AI opens doors to improving inhalation medicines
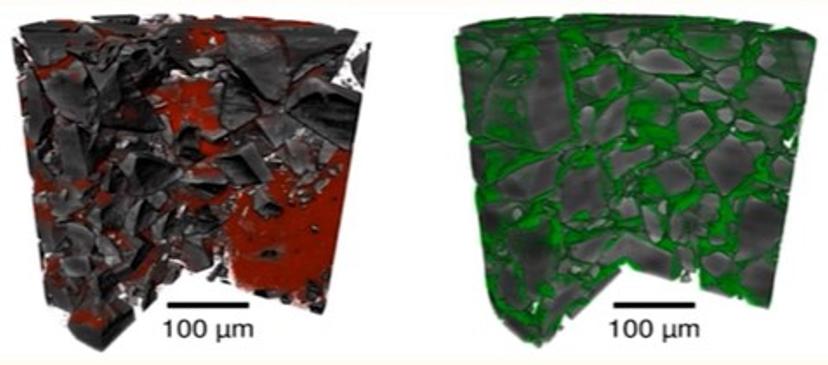
This novel ability to identify microstructural ‘fingerprints’ of APIs within powder blends has the potential to help optimize DPI formulations and provide a new method for micro-structural assessment that could be invaluable for bioequivalence studies.
Using this approach, Dr. Gajjar and Prof. Murnane have already conducted follow-up studies comparing the micro-structure of different dry powder blends, revealing stark differences in content uniformity arising from the cohesive properties of blend constituents (Fig 2).
Crucially, they found that these uniformity differences can affect the performance of the dry powders during aerosolization. “The qualitative structural differences that we measure and observe using the X-ray microscopy approaches can help us understand the aerosolization and blending behavior of these powders when they’re formulated as a DPI product,” explains Prof. Murnane. “We’re able to study the segregation or content uniformity of blends and we’re able to use that to examine some of these aerosol performances.”
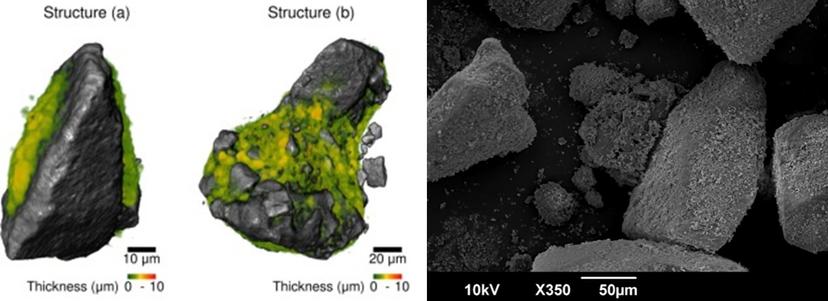
The researchers were also able to calculate the thickness of the drug-rich phases, identify individual cluster ‘units’ in bulk powders, and conduct quantitative analysis on these individual units. “Compared to the current industry standard of SEM imaging, we see a whole new depth, a much richer set of analysis that is possible with the XRM,” says Dr. Gajjar. “The AI powered tools have enabled us to look at a range of inhalation medicines in a whole new level of quantitative detail.”
Dr. Parmesh Gajjar is a Visiting Researcher from the X-ray imaging facility at the University of Manchester and Chief Technology Officer at InformiX Pharma Ltd. Prof. Darragh Murnane is a Professor of Pharmaceutics at the University of Hertfordshire and Chief Scientific Officer at InformiX Pharma Ltd.
Learn more about how correlative, AI powered X-ray microscopy can be used to quantitatively characterize asthma inhalation blends, in this on-demand webinar >>
