Seeing in 3D: How cell culture imaging just got easier
Dr. Falco Krüger discusses the challenges of 3D cell culture imaging, enabling technologies and the future of AI microscopy
19 Oct 2021
As scientists look to increasingly intricate cellular models to answer big biological questions, more advanced imaging solutions are required to obtain relevant data and make complex experiments more practical. In this exclusive interview with Dr. Falco Krüger, an Advanced Workflow Specialist for widefield microscopy at Leica Microsystems, we examine how the latest imaging and analytical methods are keeping up with this rapidly evolving field.
Falco shares some of the key challenges of 3D imaging and how state-of-the-art imaging systems from Leica are enabling researchers to utilize sophisticated methods to tackle these. He also reveals some of the exciting new developments in the field, including how artificial intelligence and machine learning look set to deliver large gains in biological image analysis.
Overcoming common hurdles
There are numerous challenges inherent to cell culture imaging. Firstly, researchers must continuously monitor and maintain the health and stability of cells to ensure data collected from observations are physiologically relevant. Multiple parameters need to be considered in order to maintain cell viability, including the content, pH and temperature of the cell media, the type of cell culture vessel, and measures to prevent contamination and phototoxicity.
At each stage of the workflow, the optimization of these factors should balance cell culture health against the desired imaging conditions. One of the key considerations here is what type of cell culture vessel to use. “If you just want to check the health and development of organoids, for example, it’s perfectly fine to use a 6-well plate that can have quite a thick plastic bottom,” says Falco. “You can use this both as a specimen holder and for imaging, and there are dedicated long working distance objectives for this.” Plastic-bottomed containers are suitable for low magnifications, however, for high-magnification immersion objectives, glass-bottomed containers with a defined glass thickness and refractive index should be used. “Here, the refractive index of the glass, as well as the mounting media for the specimen, should be matched as well as possible with the numerical aperture of the objective,” adds Falco. “This is essential for achieving the best resolution and image quality.”
Working in 3D
Three-dimensional cell culture models have emerged as important tools in life science research due to their ability to mimic the physiological state of natural tissue more accurately than their 2D counterparts. Effective imaging of 3D cell culture models, such as organoids and spheroids, however, presents its own set of challenges. How thick a specimen is, along with its light-scattering properties and level of autofluorescence, can all influence the most appropriate microscopy technique.
“All of these parameters vary depending on the application,” says Falco. “For Leica, the challenge is, therefore, to provide researchers with systems that can handle every level of work, from producing specialized images to offering very versatile imaging solutions that can tackle your everyday cell culture imaging needs.”
Supporting the entire workflow

“At Leica, we don't just want to give you a microscope, what we aim for is to assist you along every step of your workflow,” Falco continues. “When imaging organoids, for example, the final imaging can be done in seconds, but all the cell culture work before the organoid is grown takes weeks and requires careful monitoring throughout.”
During these initial stages, visual checks for confluency, cell health, and potential microbial contamination must be performed at regular intervals. “For this, researchers need routine microscopy solutions to be fast and easy-to-use,” asserts Falco. “This is where our Leica DMi1 and DM IL LED Inverted Microscopes come in.”
The Leica DMi1 and DM IL LED inverted microscopes provide different contrast methods and feature a wide range of working distances above the specimen to enable flexible imaging and easy handling of different samples. “These microscopes also allow you to stack cell flasks and look through them, which is important for the quick repetition of cell culture experiments,” adds Falco. Additional accessories, such as objectives and cameras, along with the Leica Application Suite (LAS) software, can also be equipped to meet the demands of specific applications.
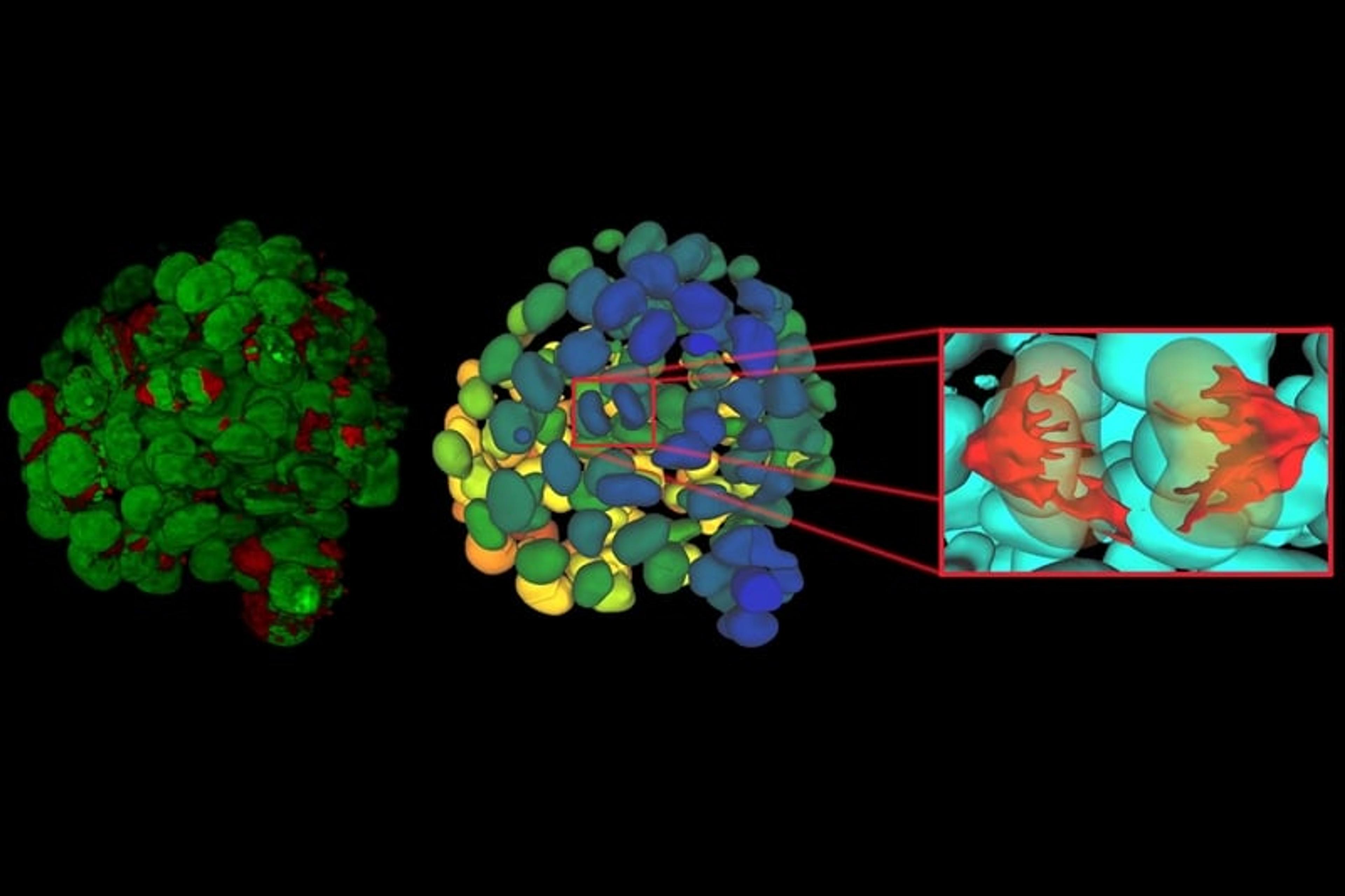
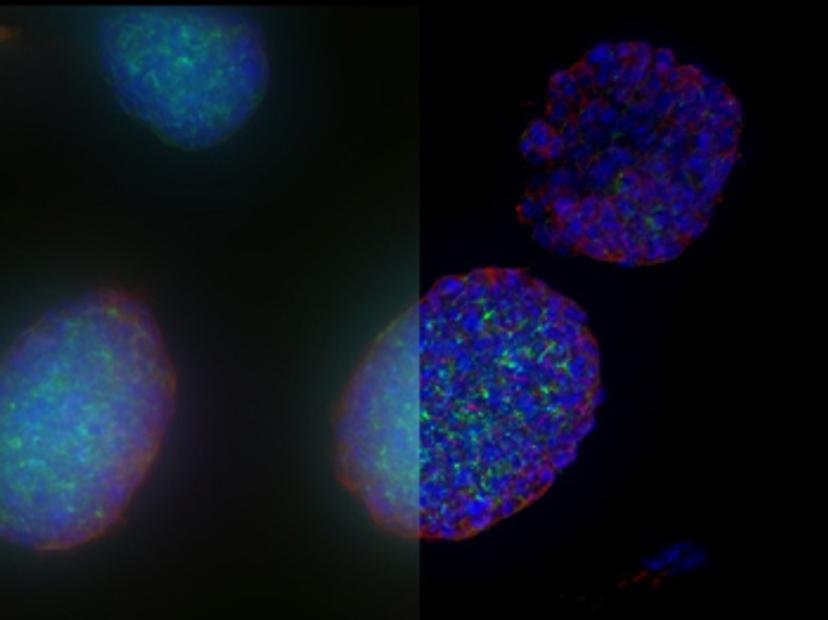
Once established, 3D cell culture models such as organoids are fixed, immunolabeled, and studied using clearing techniques to enable the visualization of their 3D structure. Usually, these studies are performed using confocal microscopes, as imaging cultures with more than 2-3 cell layers can be challenging for widefield systems, which have an inherent blur that masks the signal of interest. “This is why we introduced our family of THUNDER Imagers,” says Falco. “These provide the speed and sensitivity of a widefield microscope, but with much higher contrast, especially for 3D samples.”
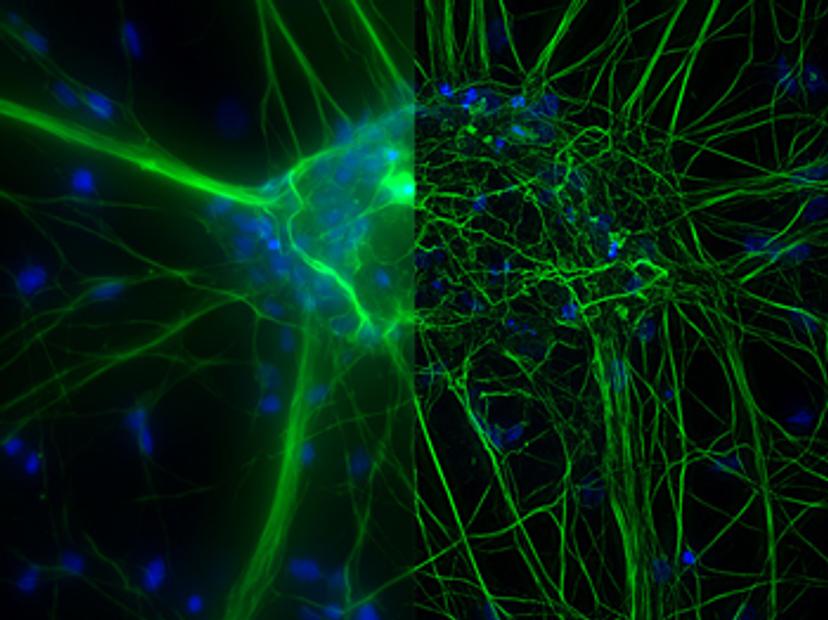
THUNDER systems feature an exclusive Computational Clearing technology which removes out-of-focus blur from areas outside the focal plane in real time. Computationally cleared images reveal details that can then be easily quantified, even deep inside thick 3D cell clusters, at high speed. “The nice thing with the THUNDER Imagers is that you always keep the raw image – meaning you can always compare your pre- and post-processed images,” Falco adds.
The type of contrast method and illumination are other critical considerations for 3D cell culture imaging. Fluorescence imaging is commonly used to visualize molecules and dynamic processes in cells but can often lead to phototoxicity and photobleaching within samples. “If you want to go for five, six, or even the seven different fluorophores in your sample, the light source needs to be as precise in excitation as possible,” explains Falco. “In THUNDER Imagers, we offer different sets of LED light sources, including a white light LED, where you select single-band filter cubes for the color you want to image, and multi-line LED, which features multiple color LEDs that allow you to flexibly excite your fluorophores.”
He continues: “As THUNDER Imagers incorporate high-end sCMOS cameras, their detection sensitivity is also really high, meaning you obtain images that are immediately suitable for analysis, even with low illumination and short exposure times. All of this increases the lifetime of both your sample and the fluorophores.”
The dawn of an AI era
With computational power ever increasing, advances in automated microscopy systems and image analysis software are driving exciting new developments in the field of microscopy. “I believe AI-powered microscopy and image analysis is really the next big thing,” says Falco. “At Leica Microsystems, we already have Aivia AI Image Analysis Software, which enables researchers to use machine learning and AI-supported image segmentation and analysis.” Using state-of-the-art, AI-first software architecture, the Aivia platform is designed to process and reconstruct highly complex images in just minutes, removing the risk of human error inherent in standard segmentation and manual curation. It means researchers can quickly and reliably generate high-quality results for publication without performing repetitive tasks that may otherwise impact image analysis. “This is an essential step on the path towards fully autonomous image analysis, and I believe we will see more AI features implemented in microscopy platforms so that researchers can make use of this powerful solution,” Falco concludes.
Do you use Leica Microsystems products in your lab? Write a review today for your chance to win a $400 Amazon gift card>>