Best practices to transform lateral flow assay development
Lateral flow assay tests are essential tools that provide critical information across various applications. These tests enable accurate and timely detection of infectious diseases, pathological conditions, and metabolic states, facilitating informed decision-making in diverse fields. Access to reliable point-of-care tools is vital for ensuring optimal outcomes, whether in clinical settings, environmental monitoring, or food safety. Recent technological advancements have paved the way for innovative lateral flow assays, allowing for enhanced accuracy and speed in testing, ultimately benefiting end-users and stakeholders across multiple industries.
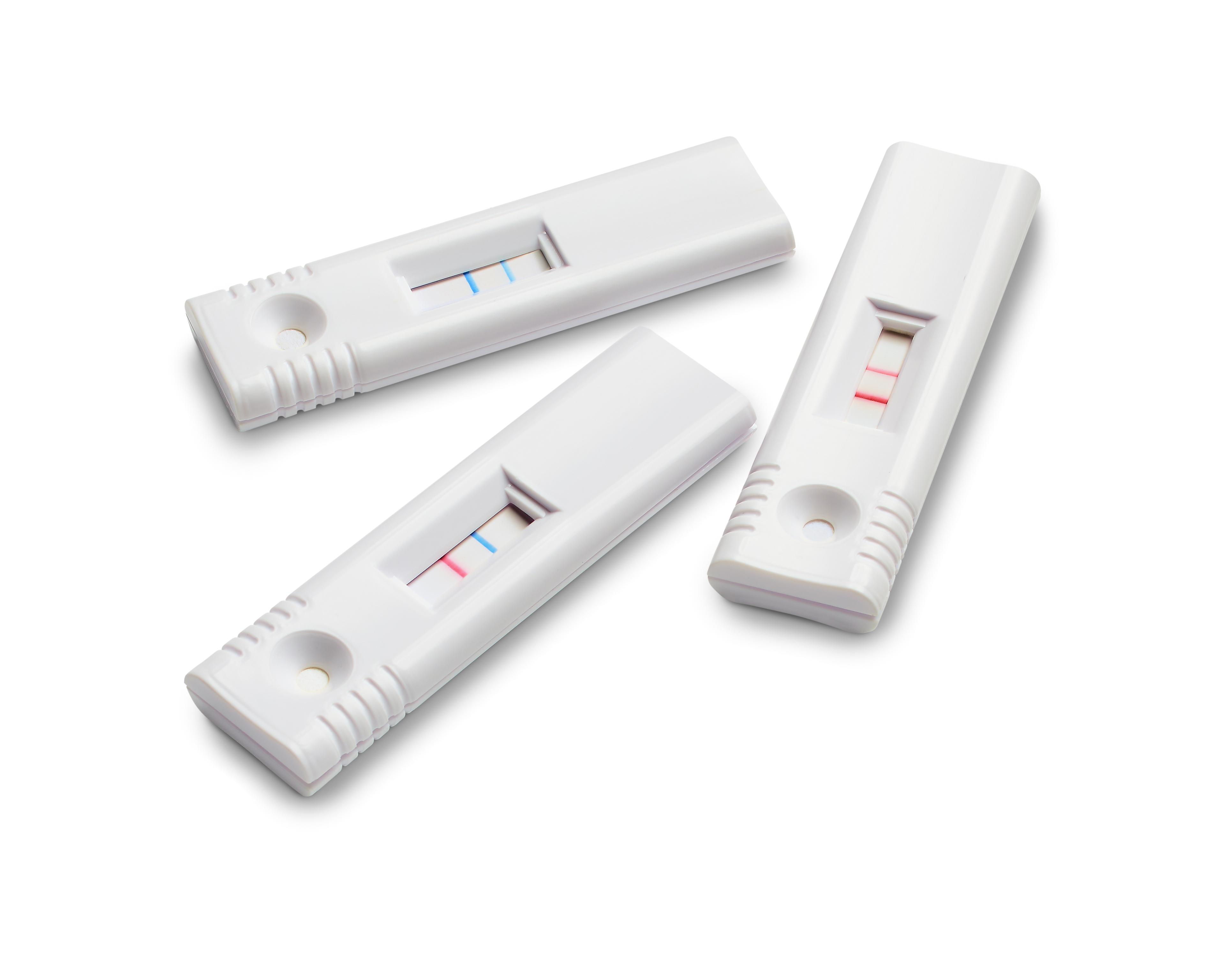
One of these many diagnostic tests is the lateral flow test strip. Within the wide range of current applications, these easy-to-use diagnostic devices are used to confirm ovulation and pregnancy in women, detect viral and bacterial infections in humans and animals, quantify biomarkers in human body fluids, determine the presence of pathogens in food, and screen for contaminants in environmental samples. Lateral flow tests became much more familiar to the general public during the COVID-19 pandemic, even though they were routinely used long before the outbreak. These immunoassays will continue to be regularly used for rapid testing across a range of industries – from environmental analysis and food testing to pharma and diagnostics.

Dr. Katie Spitere, former Product Portfolio Manager, Membrane Applications and Rapid Technologies, Luis Manuel Granados, Scientific Sales Specialist Immunoassay and Molecular Raw Materials, Sarah Nadin, former Head of Assay Development Services, and Roy Wu, Managing Director of Merck Life Science in China discuss how Merck is working to shape the future of rapid diagnostics by advancing lateral flow tests and ensuring preparedness for future pandemics.
Read article
For a deeper understanding of common challenges faced during the development of lateral flow tests and effective strategies to overcome them, explore this article as it provides valuable insights that can enhance your approach to assay development and troubleshooting.
Read article
In vitro diagnostics
Healthcare providers rely on a variety of diagnostic tools to help them accurately diagnose and monitor conditions, which, in turn, helps to better guide treatment and management decisions. Among the most used diagnostic tools are in vitro diagnostics (IVD). IVDs are clinical tests performed on samples taken from the body, such as blood or tissue. These tests are vital for accurate disease detection and prevention, along with monitoring a person’s overall health. The term in vitro simply means ‘in glass’, which signifies that this test is conducted in a test tube, as opposed to in vivo tests, which are executed within the body.
IVDs can be performed via a range of different tools, from handheld devices to more complex, large, and sophisticated laboratory instruments. For instance – handheld and mobile diagnostic devices can be particularly valuable for healthcare facilities that reside in low- to middle-income countries, where laboratory access is limited, and healthcare can be compromised. One of the most valuable of these many tests is the lateral flow test, which became apparent during the COVID-19 pandemic and created a surge in their demand. As more demand is created for these types of tests, IVD manufacturers are challenged with an ever-changing regulatory landscape. It is important that the correct raw materials are chosen to best support their needs.
In the resources below, we explore IVD manufacturing and reveal how to best select high-quality raw materials when building a dependable IVD assay.




The diagram below represents the different stages of the assay development process.
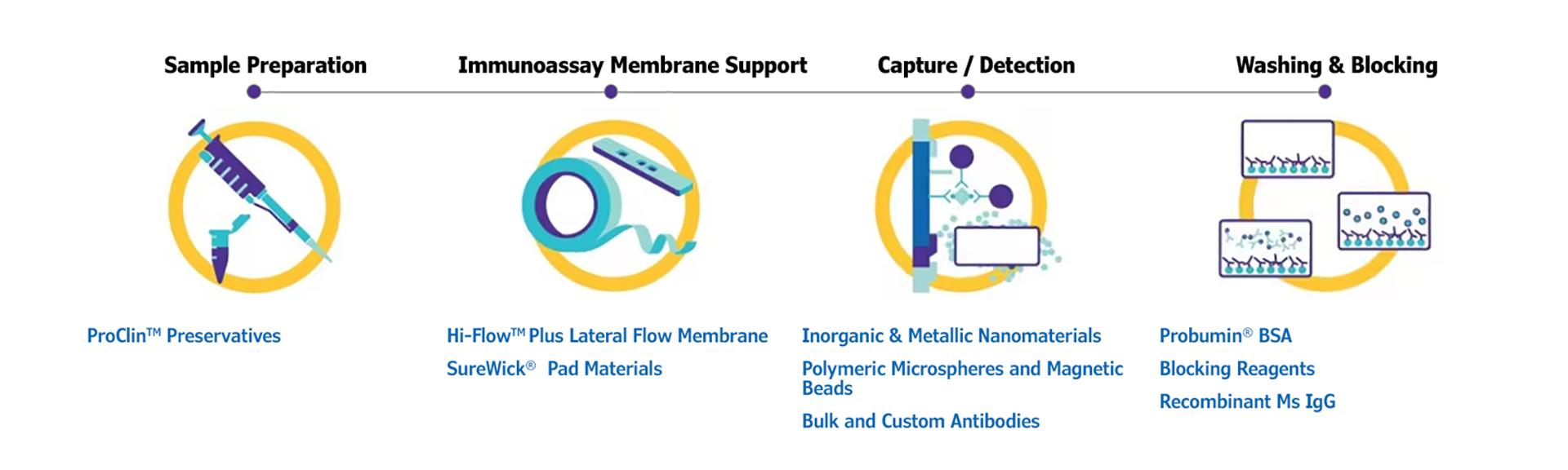
Scientific partnerships bringing big results
In 2021, Merck KGaA Darmstadt, Germany, was awarded a €121 million contract from the United States government for the construction of a lateral flow membrane production facility to support the surge in demand experienced by its IVD customers. Merck KGaA is a leading science and technology company that has gained a positive worldwide reputation for its range of high-quality diagnostic solutions. Read press release here.
Looking to validate membrane for a new project in the United States? Inquire with your account rep about the enhanced flexibility and supply security offered by the Hi-Flow™ Plus lateral flow membranes and the new production facility in the United States.

Complete solutions
Making the jump from concept to clinic
Developing robust and sustainable diagnostic tools can be a long, expensive, and complex process. To allow for innovative diagnostic tools to move effectively from concept to clinic, it is essential that manufacturers collaborate with trusted partners who understand the complexities of the diagnostic market. The experts at Merck KGaA offer more than state-of-the-art product lines and reagents, for COVID-19 detection they also work closely with their customers on regulatory compliance, raw material sourcing, quality control, manufacturing, and production, to help them deliver consistent results. Within this immersive article, we will highlight the solutions Merck KGaA offers that help manufacturers streamline their transition from concept to clinical practice.
Membranes and pad materials
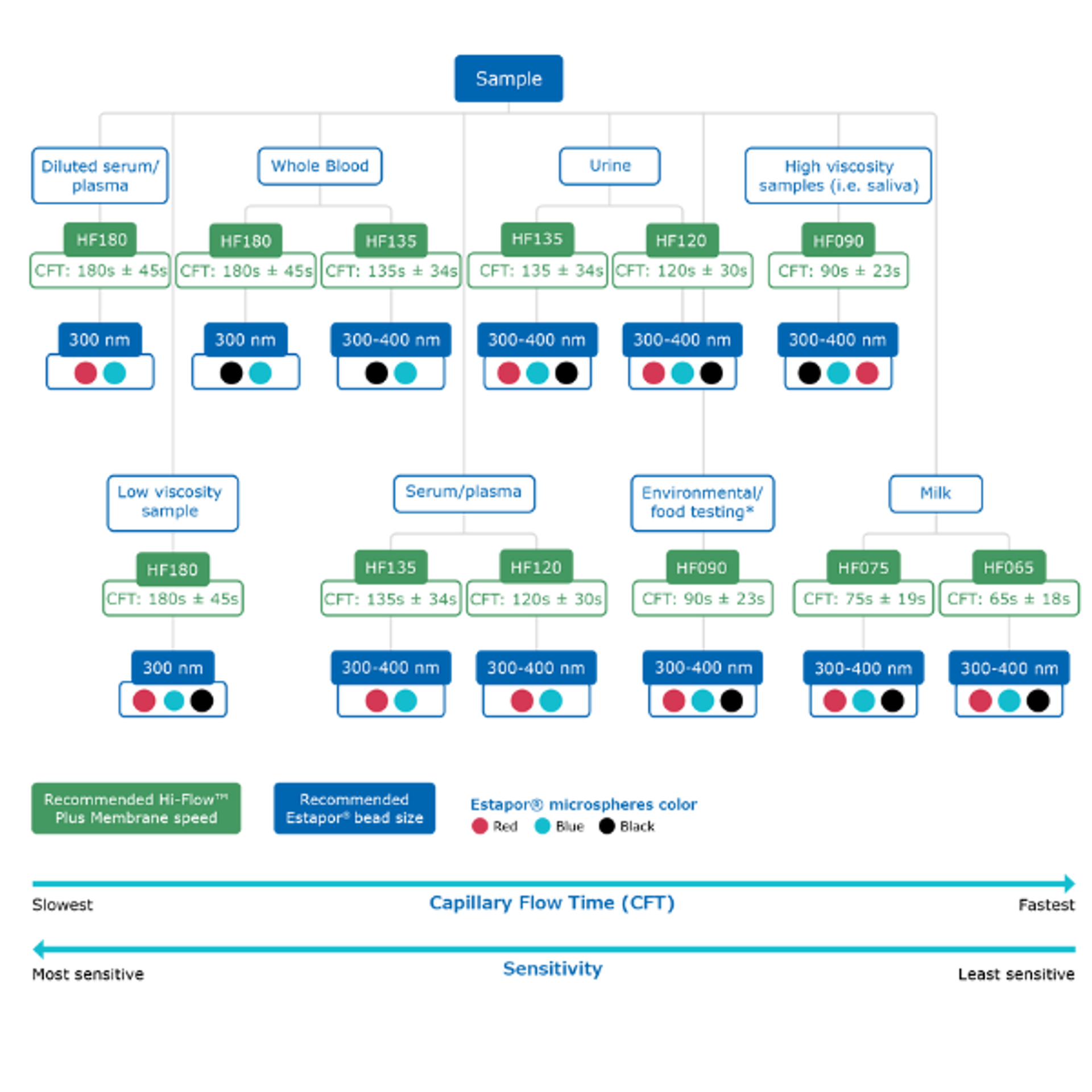
Lateral flow membranes are essential for rapid diagnostic test kits. For over 30 years, Merck KGaA has developed Hi-Flow™ Plus Lateral Flow Membranes, available in formats like rolls and cards, known for their superior consistency and performance.
Choosing the right membrane is essential for optimal assay performance. Hi-Flow™ Plus membranes offer various flow rates and sensitivities to accommodate different sample types and testing requirements. Important factors to consider when selecting a membrane include:
- Compatibility with sample types
- Capillary flow rate
- Surface quality
- Membrane composition
- Reagent usage
Hi-Flow™ Plus membranes are optimized for lateral flow assays, manufactured to tight specifications for reliable lot-to-lot performance, fixed test run times, and minimal nonspecific background signals. They are suitable for all lateral flow test types and come in various speeds to meet specific application needs. For instance, fast-flowing Hi-Flow™ Plus 65 membranes are ideal for speed-critical tests, while slow-flowing Hi-Flow™ Plus 180 membranes are suited for high-sensitivity applications.
Available Formats:
Sample Packs:
- Variety Sample Sheet Packs: A selection for early evaluation
Request your free Variety Sample Pack
- Sample Sheet Packs: Five sheets for initial test development.
- Sample Slit Rolls: Individual rolls for later development stages.
Manufacturing Options:
- 20mm and 25mm Wide Slit Rolls: Offered in various capillary flow speeds and backing options, available in convenient packs for immediate shipping.
- Membranes are also available to custom order to the slit width of choice.
Preassembled Hi-Flow™ Plus Membrane Cards provide an efficient option for manufacturers, eliminating the need for manual assembly and ensuring consistent test performance.
To help support users further, Merck KGaA has also created the SureWick® Conjugate, Sample, and Absorbent Pads to provide a complete solution for lateral flow test strip manufacture, which ensures that consistency of performance is achieved with each test strip. These materials are available in strip, sheet, and roll formats to suit different manufacturing processes.
In the resources below, learn how to simplify assay development, and accelerate the time to get to market with fit-for-use products to meet your IVD development and manufacturing needs.





Microspheres
Estapor® microspheres are small organic or inorganic spherical particles with a diameter of around 1 μm to 1000 μm. These modest particles encompass a significantly large surface area, which helps to generate faster kinetics and lower detection limits. Interestingly, their surfaces can be modified with different surface chemistries for specialized applications, such as to be used within diagnostic assays, flow cytometry, labeling, and much more. Microspheres can be used in many different applications such as lateral flow, CLIA, and LTIA.
Microsphere-based assays deliver valuable quantitative and qualitative information to clinicians, and Estapor® microspheres are used in a variety of applications, from diagnostic screening and immunoassay development to targeted drug delivery. In the case of lateral flow development, these particles improve assay sensitivity through fluorescence detection, make assays easier to read, and allow for signal quantification. However, there needs to be a balance between sensitivity and assay run time, which should be considered when selecting the right microspheres to meet your needs. For instance, larger microspheres increase assay sensitivity in exchange for a longer assay run time. To help get the balance right, Estapor® microspheres have been one of the top choices for many lateral flow diagnostic test manufacturers. These microspheres have been developed to be easily characterized and designed with key features to meet your needs.

Discover the power of multiplex assays with advanced Estapor® Intense Microspheres. These innovative microspheres are engineered to enhance the functionality of lateral flow tests, enabling the simultaneous detection of multiple analytes on a single test strip. Each analyte is easily identifiable with microspheres in red, blue, or black, improving clarity and simplifying interpretation. Estapor® Intense Microspheres offer significant advantages for diagnostic applications. They enhance multiplexing capabilities by incorporating multiple test lines on one strip, each marked by a distinct color. This not only provides clear visual results but also minimizes the risk of data misinterpretation, ensuring thorough and reliable testing. Performance comparisons of Estapor® Red Intense microspheres against recognized competitors in a model HBsAg antigen assay are detailed in the Estapor® Red Intense Microspheres Performance in Lateral Flow Assay Report. Moving beyond the traditional colloidal gold standard, known for visibility but limited to single-analyte detection, Estapor® Intense Microspheres facilitate complex, multiplexed assays. This innovation allows for a wider range of testing while maintaining ease of result interpretation.
Explore the resources below for protocols on covalent coupling of proteins to Estapor® Microspheres and their utilization and optimization in lateral flow tests:






Discover the technology for all your lateral flow needs

Get expert support here
Technological advances have allowed for rapid diagnostic tools to take testing away from the laboratory and move it closer to the patient, whilst also delivering an accurate and timely result. Since we are bringing the test to the patient, it is critical to ensure consistent, robust, and high-quality materials are used for lateral flow assay development. Researchers must ensure they select the most suitable reagents, membranes, and antibodies to optimize their tests.
Assay development services are now availableEssential lateral flow products
Sheets
Sample slit rolls
Membrane cards
Sample and absorbent pads
SureWick Sample and Absorbent Pads
SureWick Glass Fiber Diagnostic Pads
Estapor Functionalized Microspheres
Estapor Microspheres

Quality resources in lateral flow testing
Merck KGaA offers a new assay development service that works with you to optimize your lateral flow assay project. As a raw materials supplier, Merck KGaA understands the importance of getting the components right the first time, and aims to be a valued partner for successful test to market. With laboratories and expertise in both the US and Europe, Merck KGaA has consultants who are ready to help you optimize your project and expedite your time

to market.
Benefits of working with a lateral flow membrane expert:
- Utilize vast knowledge for sourcing products
- Leverage R&D scientists with dedicated laboratories for troubleshooting
- Avoid common IVD assay development pitfalls
- Learn how to accelerate commercialization
- Increase confidence in response to regulatory challenges
- Reduce time and supplies in optimization steps
To find out more about this expert service, hear from Eileen Hannigan and Sarah Nadin at Merck, as they share the benefits of working with a raw material supplier who also supports lateral flow assay development.
Learn how to optimize lateral flow assays for successIn this on-demand webinar, explore what goes into an effective lateral flow test. This webinar will focus on the critical factors involved in optimizing test strips as well as the importance of consistent manufacturing processes and reagents to ensure accuracy and reliability. Plus, Dr. Hannigan will explain how attention to detail in material selection, chemistry, and production can minimize variation in the final product, ultimately enhancing test performance.
Watch on-demand the essential elements of lateral flow design*Certain images and/or photos on this page are the copyrighted property of 123RF.com, its contributors or its licensed partners and are being used with permission under the relevant license. These images and/or photos may not be copied or downloaded without permission from 123RF.com. Other images courtesy of Merck KGaA.