News & Articles
DeNovix CellDrop Automated Cell Counter receives second major accolade of 2020
The DeNovix CellDrop™ Automated Cell Counter has been awarded the Gold Seal of Quality based on outstanding scientist reviews
Mogrify introduces platform to identify epigenetic switches driving cell identity and cell maintenance
EpiMOGRIFY supports the development of scalable off-the-shelf cell therapies for diseases with a high unmet clinical need
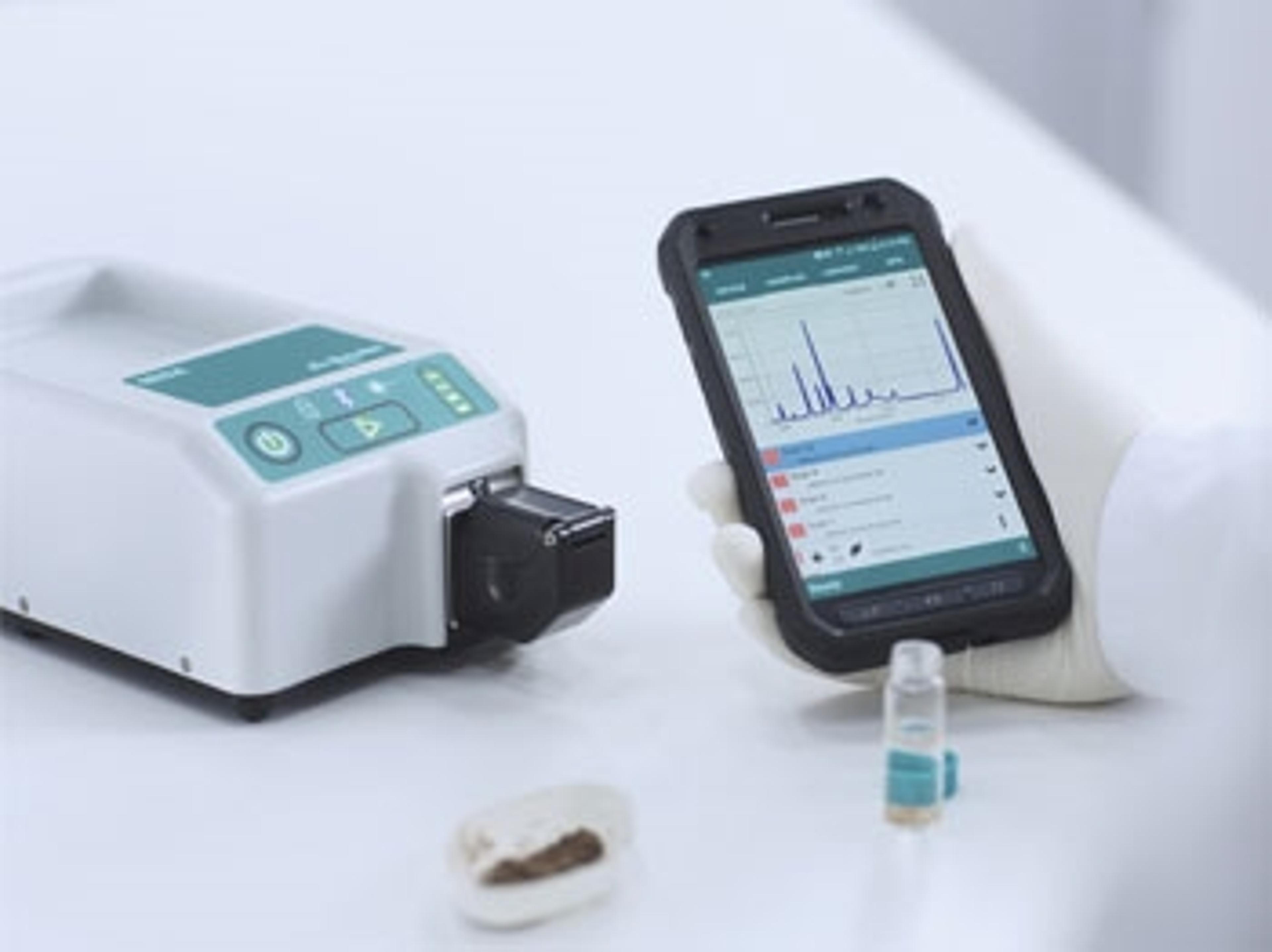
Metrohm launches Misa – a new, portable food analysis system
New product is designed to protect consumers with the latest in food testing technology
New special feature: Advance your 3D cell culture research with these top resources
Explore exclusive interviews, new methods, and free download to help optimize your 3D cell culture
Simplify volatiles analysis (Part 3): Method development and validation for direct MS
Join us on Tuesday, October 27, to learn more about how SIFT-MS works, how it speeds up analysis, and how it can be validated successfully in accordance with ICH Q2(R1) Guidelines
Revolutionize your immunoassay development with SpyTag antibodies
Join us on Wednesday, October 28, to learn how SpyTag-SpyCatcher technology enables site-directed conjugation and rapid assembly of different antibody format
GenMark Diagnostics’ ePlex Respiratory Pathogen Panel 2 Receives EUA from FDA
Combination test for COVID-19, flu and other common respiratory illnesses helps health care professionals prepare for flu season
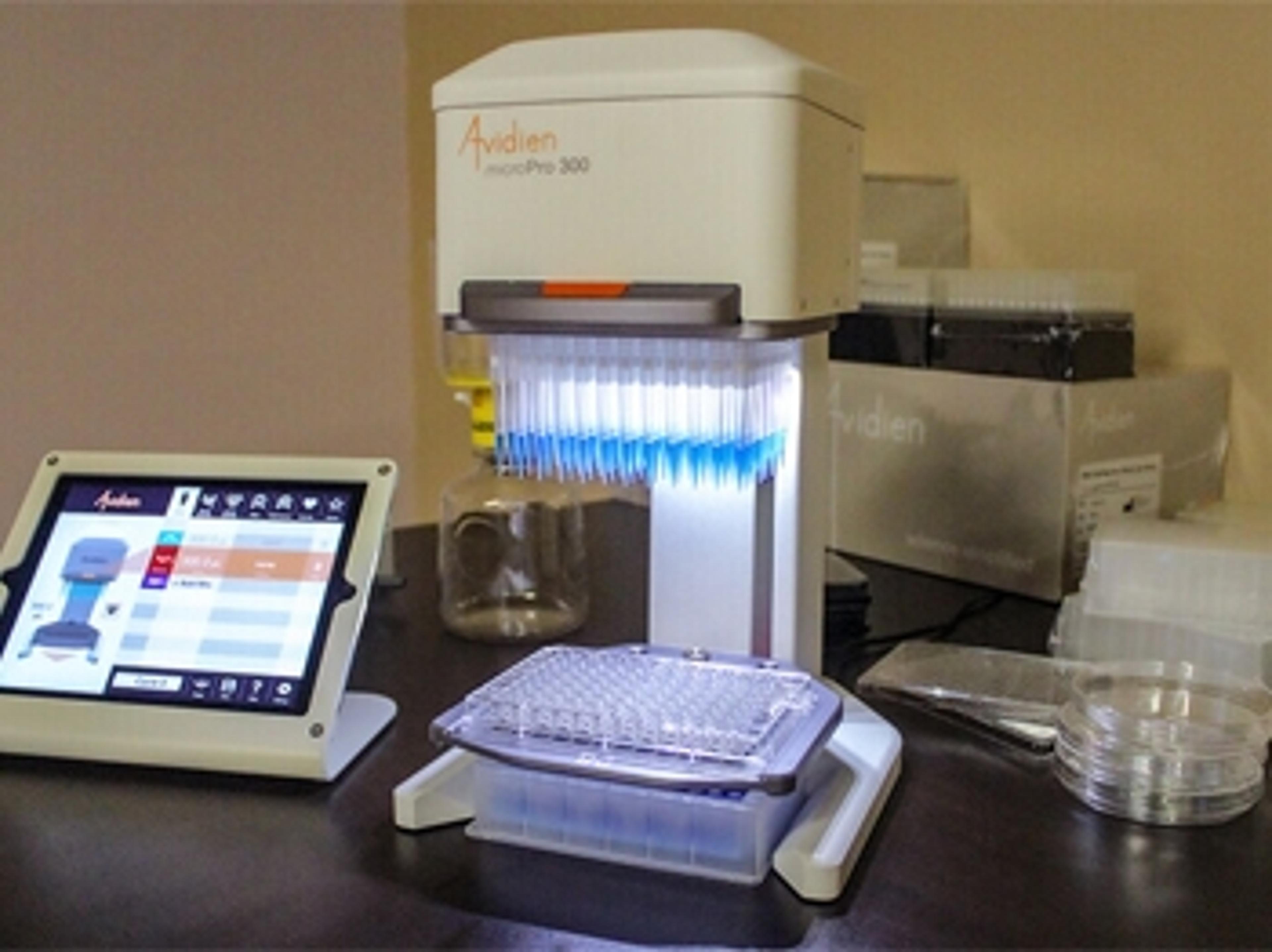
MicroPro in the Lab: Avidien announces winner of microPro giveaway contest
For their prize, Dr. Lieberman and her team have chosen to receive the upcoming low volume model, the microPro 25
University of Minnesota opens clinical trial to treat metastatic GI cancers using CRISPR genetic engineering
The Phase II clinical trial, which will use cutting-edge CRISPR genetic engineering, has already begun enrolling patients
PerkinElmer launches fully automated workflow for cannabis & hemp pesticide residue testing
Streamlined solution features quality control reagent kits, robotic sample preparation, mass spectrometry analysis and data reporting software to transform analysis efficiency
Thermo Fisher Scientific further expands COVID-19 test portfolio with two new antibody tests
The addition of COVID-19 antibody tests to the portfolio aims to further support the pandemic response on multiple fronts
Most loved by scientists: 5 top-rated lab products awarded new Seal of Quality
We reveal the cell counters, LC systems and particle size analyzer that have earned one of these coveted awards based on exceptional reviewer feedback
New methods to rapidly evaluate virus-neutralizing antibodies
Learn how high-throughput, real-time cell analysis can accurately detect and quantify virus-neutralizing antibodies and see how this technology is advancing COVID-19 research
Getting to the root of the problem: How single-cell RNA sequencing is helping to improve crop yields
Find out how understanding the pathways involved in root growth inhibition can help address one of the biggest challenges in agriculture
Ambient ionization mass spectrometry in space and time: From high throughput analysis to molecular imaging
Join us Thursday, October 22, to explore the applications and benefits of ambient ionization for biomedical research and its future potential for pharma and food industries
Mologic launches rapid diagnostic test for fungal crop pathogen Botrytis
BotrytisAlert harnesses Mologic’s expert lateral flow technology to enable earlier disease intervention and prevent wide-spread crop failures
Beckman Coulter completes first in a series of antibody neutralization studies
Access SARS-CoV-2 IgG assay shows excellent agreement with the Genscript Surrogate Virus Neutralization Test
Joining forces against SARS-CoV-2: Rentschler Biopharma contributes to manufacturing of COVID-19 mRNA vaccine
Rentschler Biopharma to provide cGMP manufacturing services for mRNA-based vaccine candidate, developed by BioNTech and Pfizer against SARS-CoV-2
Severe COVID-19 infection linked to overactive immune cells
Samples from the lungs of patients show a runaway immune system reaction could be one mechanism behind severe COVID-19 cases