News & Articles
Selected Filters:
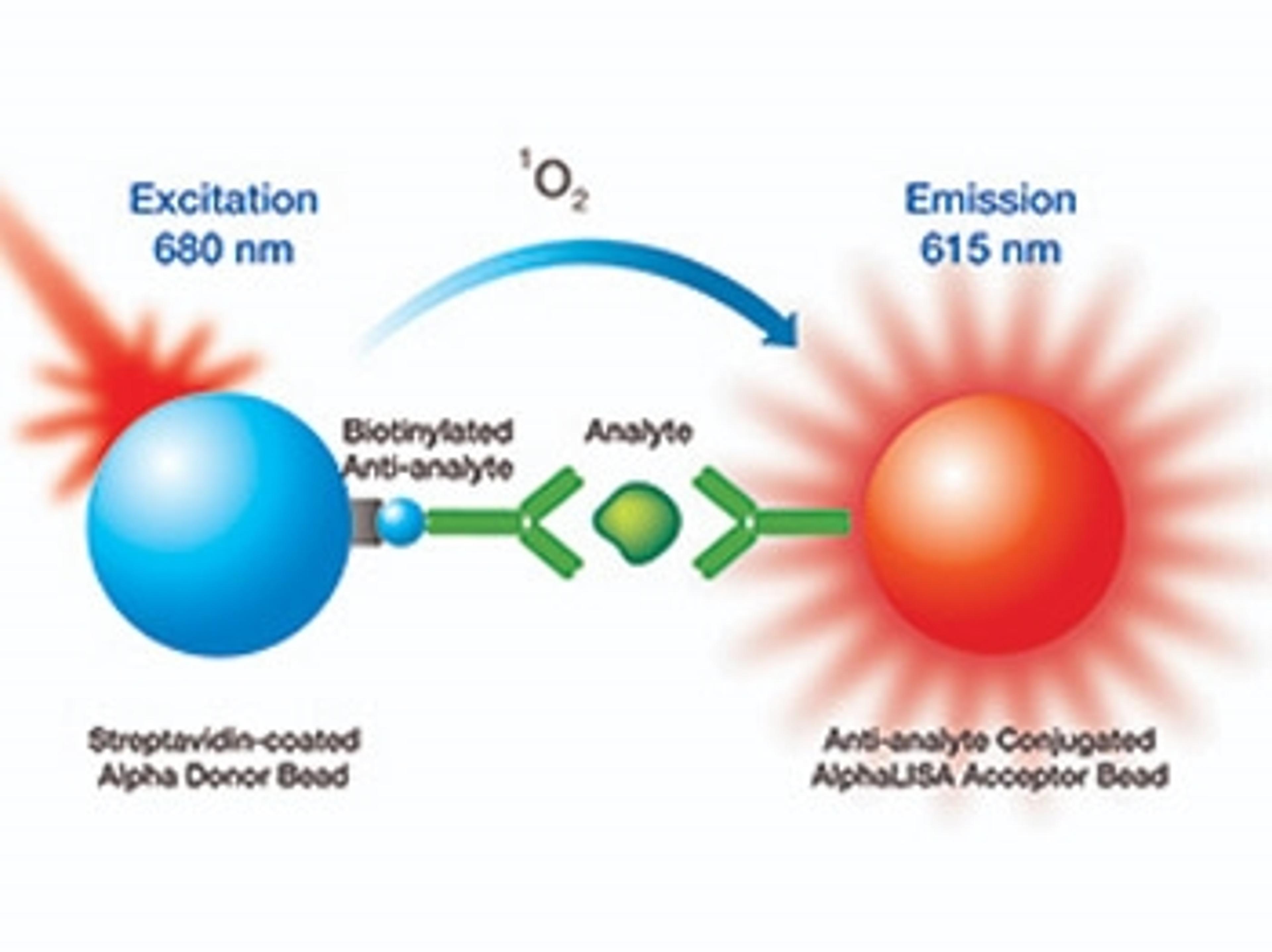
Study using Mission Bio’s Tapestri platform reveals clonal diversity underlying AML and therapy resistance
The study leverages the Tapestri Platform to illustrate the single-cell atlas of acute myeloid leukemia (AML) with multi-omics, describing the clonality underlying therapeutic resistance
Merck’s new VirusExpress platform speeds development of cell and gene therapies
Proven, scalable platform aims to increase dose yields and reduces process development time for cell and gene therapies
Pacific Biosciences and Invitae to develop whole genome sequencing-based assays for pediatric epilepsy diagnostics
The collaboration will focus on the investigation of clinically relevant molecular targets for use in the development of advanced diagnostic testing for epilepsy
FDA approves Gilead's antiviral remdesivir drug for treatment of COVID-19
Veklury is first FDA-approved treatment for COVID-19 in the US, and aims to shorten time to recovery by five days
Roche announces collaboration with Atea Pharmaceuticals to develop a potential oral treatment for COVID-19 patients
Roche and Atea partner to jointly develop AT-527, an orally administered direct-acting antiviral (DAA) currently in Phase 2 clinical trials
Virtual Neuroscience Summit: Registration now open
Watch world-renowned experts and connect with leading innovators on the hottest topics, new developments and latest technologies in neuroscience
Study shows novel mass screening method could slash COVID-19 testing costs
The hypercube algorithm aims to enable regular, cost-effective screening in multiple contexts
Teknova begins distributing COVID-19 transport media after completing FDA notification
The company aims to meet the increased demand for approved media to maintain virus viability from sample collection to the lab
Base editing: Impact on cell therapy and how to access it
Join us Thursday, November 5, to learn how to introduce base editing technology into your cell therapy pipeline
Thermo Fisher Scientific increases availability of COVID-19 testing with sample collection kit from Everlywell
The TaqPath test is authorized for use with the Everlywell COVID-19 Test Home Collection Kit, which enables individuals to self-collect nasal swab specimens
Limit of detection: The key to preventing false negative COVID-19 tests, regardless of patient viral load
Learn the importance of low LoD tests and discover the most sensitive kits available, as determined by the FDA’s SARS-CoV-2 Reference Panel
From sensitive detection to automated data management: Explore how Zymo Research is streamlining COVID-19 testing
Find out how Zymo Research’s state-of-the-art tools are helping laboratories and government agencies facilitate large-scale COVID-19 testing in the United States
COVID-19 breakthroughs: How pioneering scientists are advancing antibody therapies
Experts share novel techniques for accelerating monoclonal antibody discovery and development to bring hope to COVID-19 patients
The role of aberrant cytokine activity in the host immune response to COVID-19
Watch this webinar on demand to learn about the host immune response to COVID-19
Biomarker discovery for lysosomal storage disorders using a metabolomic approach: Your questions answered
Watch this on-demand webinar to learn how to perform untargeted/semi-targeted metabolomic studies to discover novel biomarkers by mass spectrometry
MIS-C detection: Beckman Coulter partners with BARDA in rapid diagnostic for pediatric complication of SARS-CoV-2
The partnership aims to expand existing Monocyte Distribution Width (MDW) hematology-based biomarker to potentially aid in the rapid detection of MIS-C
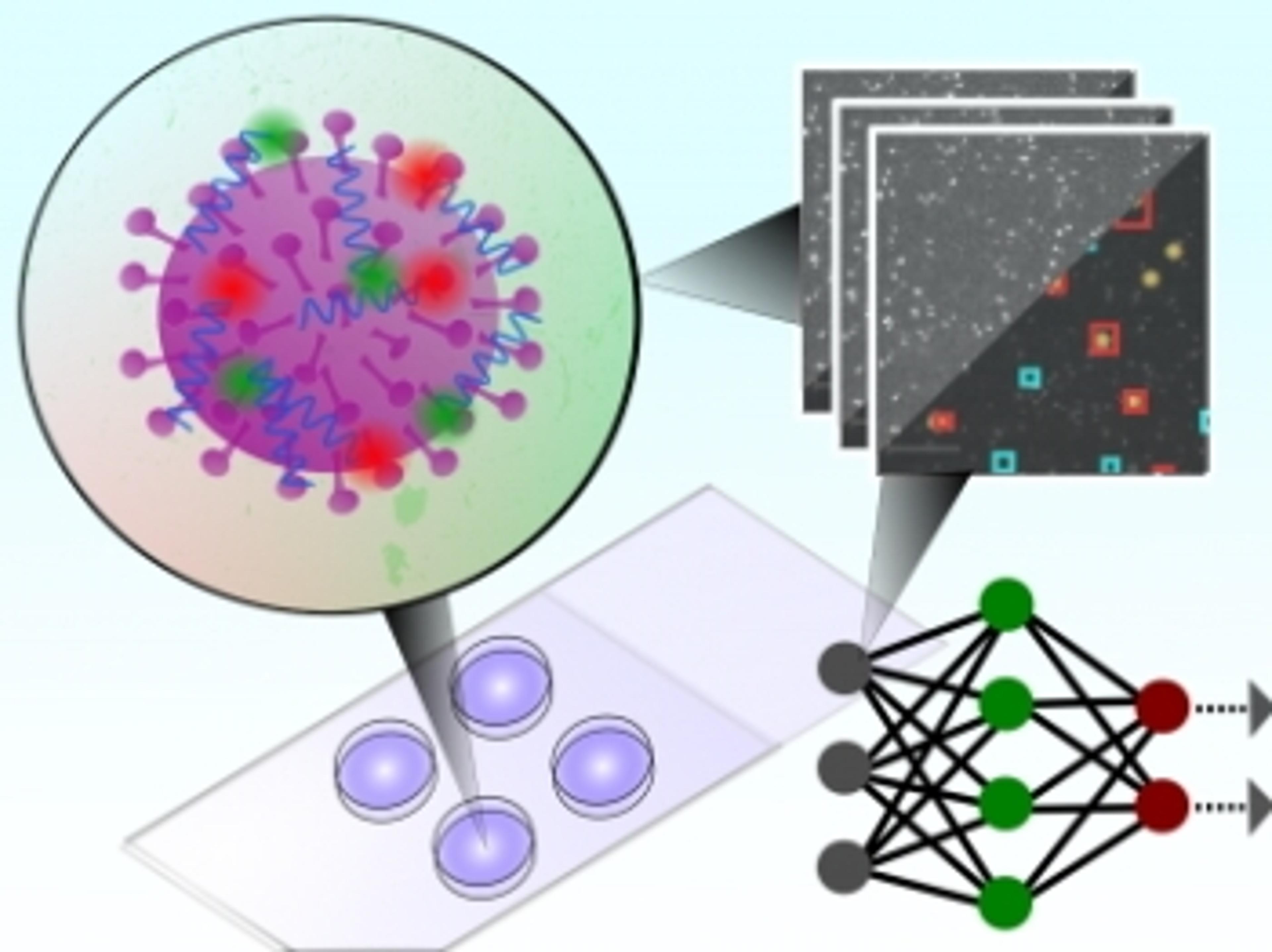
Oxford scientists develop 5-minute COVID-19 antigen test
The test uses a convolutional neural network to classify microscopy images of single intact particles of different viruses
Roche to launch lab SARS-CoV-2 antigen test to support high-volume testing of suspected COVID-19 patients
In combination with other COVID-19 diagnostic tests, the Elecsys SARS-CoV-2 Antigen test can help in the management of patients for optimal care delivery
Abbott receives FDA emergency use authorization for its COVID-19 IgM antibody blood test
Availability of the new IgM blood test on the ARCHITECT and Alinity platforms is part of Abbott's effort to offer tests across the disease progression of COVID-19
Siemens Healthineers launches rapid antigen test for the detection of SARS-CoV-2
Easy-to-use test, which doesn't require specialized laboratory personnel or instruments, promises flexibility to test in locations that benefit from immediate results
Thermo Fisher Scientific launches modular closed cell processing system for cell therapy manufacturing
The CTS Rotea system aims to help researchers overcome manufacturing hurdles and bring the vast potential of cell therapies to more patients