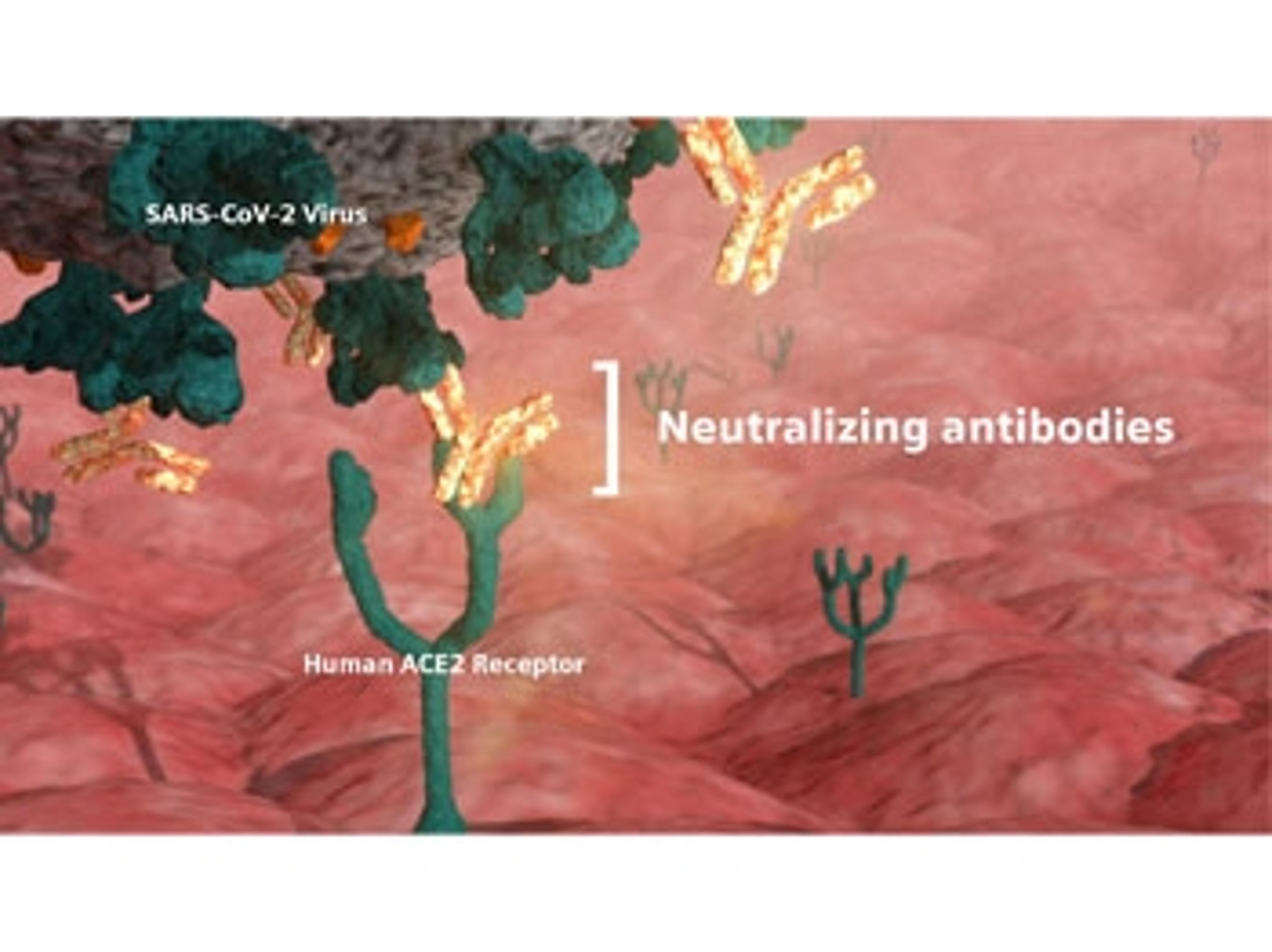
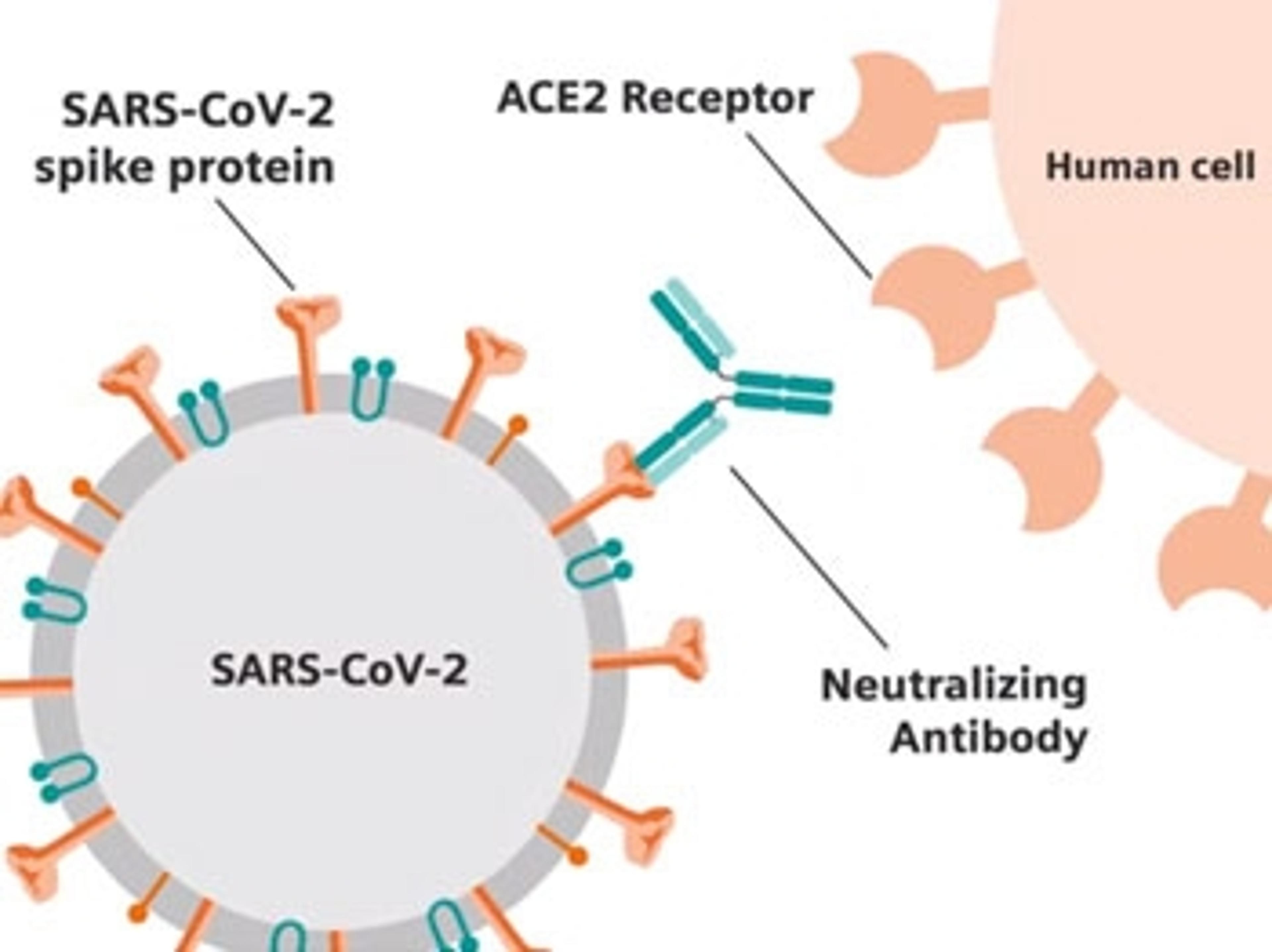
Close the distance between providers and care with human-centered engineering in the lab
At the ADLM 2024 Clinical Lab Expo (July 28–August 1, Chicago, Booth #501), Siemens Healthineers will demonstrate what is achievable for laboratory testing with human-centered engineering and automation.