Microwell plates to standardize automated organoid workflows
Gri3D® is an all-in-one microwell plate allowing researchers in drug development and academia to establish and automate front-to-end 3D cell culture workflows.
26 Sept 2024Gri3D® is a specialized microwell plate technology developed by SUN bioscience (Lausanne, Switzerland) for 3D cell culture. It is designed to support the growth and differentiation of organoids and spheroids, enabling researchers to create and study complex 3D cell models that closely mimic in vivo tissue environments. The plates are equipped with cell-repellent hydrogel microwells that confine the growth of these 3D cell structures, ensuring uniformity and consistency in experiments, and a pipetting port to allow for medium changes without disturbing the 3D cell structures in those microwells1. Gri3D® plates are compatible with a wide range of assays and imaging systems, making them a versatile tool for applications in drug discovery, toxicology, and other areas of biomedical research.
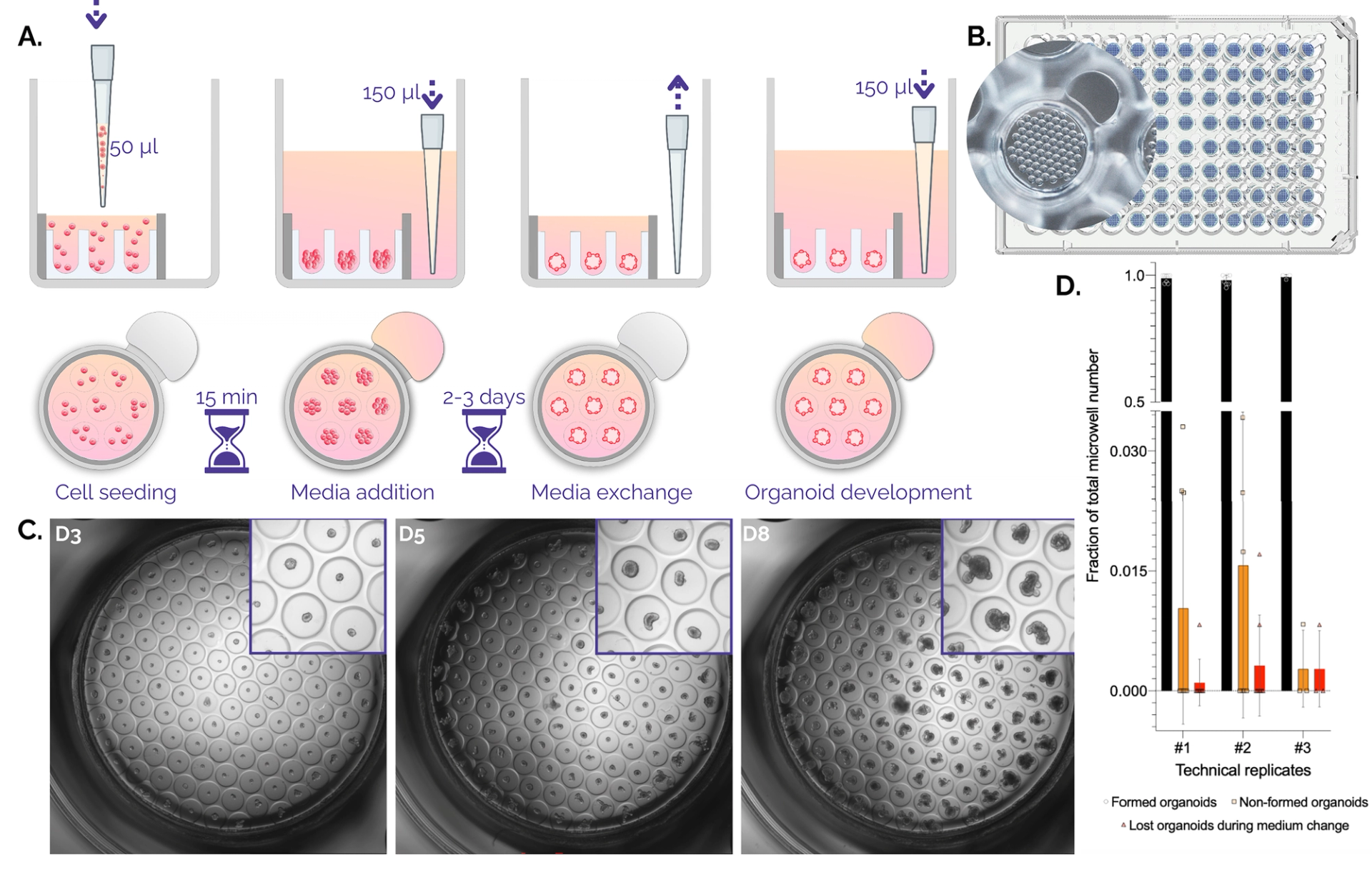
Figure 1. Organoid culture in Gri3D®microwell plates. A. Schematic workflow on Gri3D 96WP plates. B. Top view render of a Gri3D® microwell plate with zoom on one well with a 500-micrometer microwell array. C. A 4X scan of mouse intestinal organoids grown on Gri3D® at days three, five, and eight after one, two, or three medium changes respectively. D. Quantification of organoid-formation efficiency. Non-formed organoids and lost organoids due to medium changes account on average for less than 5% of the total amount of microcavities. Data are mean ± s.d. Technical replicates 1, 2, and 3 comprised n = 8, n = 8, and n = 3 biological replicates.
Error-free handling, even for beginners
We spoke with Dr. Özlem Vural, Scientist in Microphysiological Systems at Bayer AG, about their pioneering work and the essential role of Gri3D® in advancing studies of drug toxicity in primary human liver spheroids. Dr. Vural uses Gri3D® microwell plates for cytotoxicity assays on 3D primary human liver spheroids, integrating them with CellTiter-Glo® and various image-based assays2.
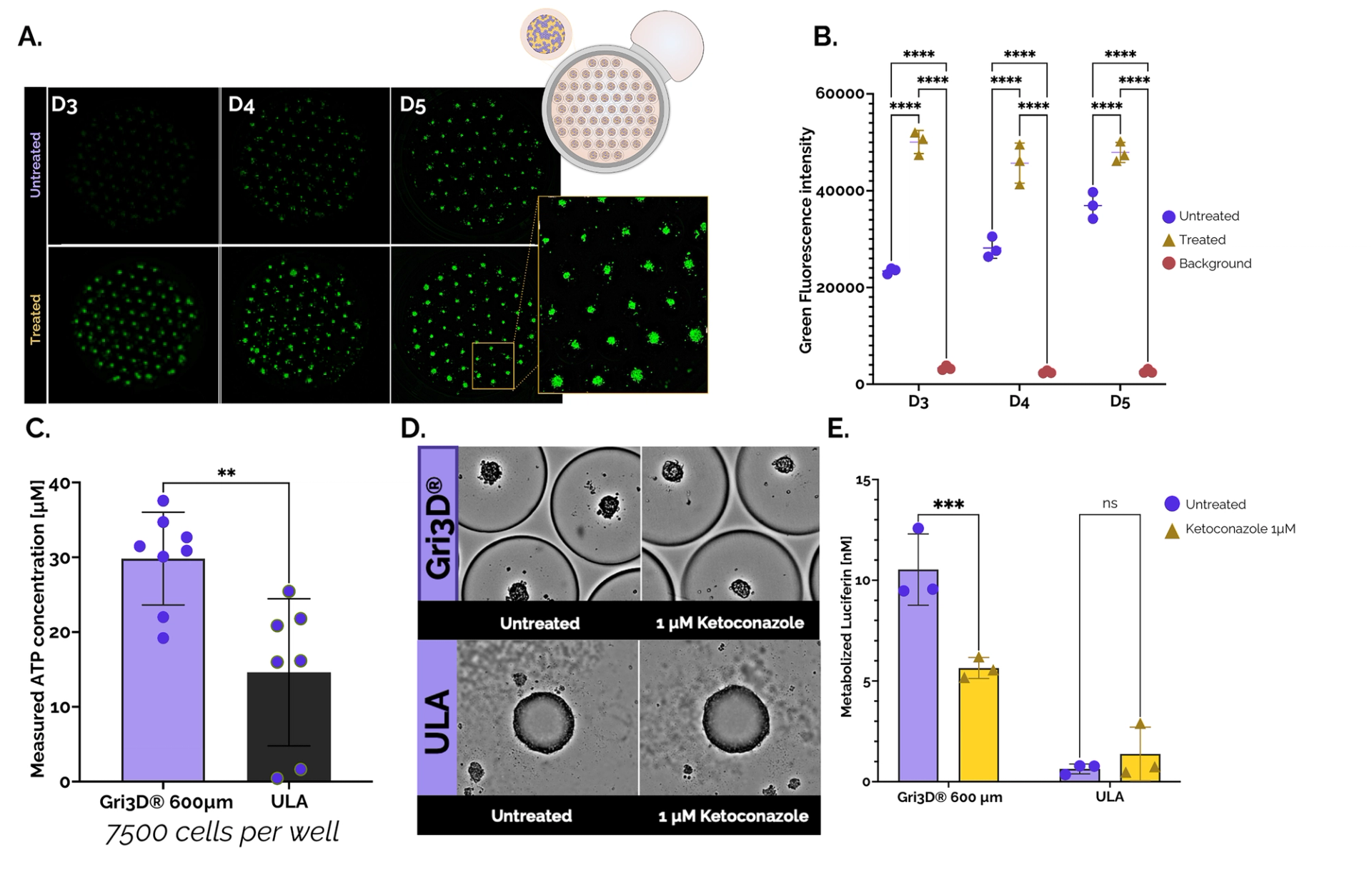
Figure 2. Cytotoxicity and CYP3A4 activity of Upcyte® hepatocyte spheroids grown in Gri3D 96WP 500 µm black-walled or ULA 96WP plates. A. Images from Spark® Cyto (Tecan) depicting untreated and treated (Triton-X) Gri3D® arrays at days three, four, and five (CellTox TM Green, Promega). B. Cytotoxicity quantification on-plate. C. Viability of primary hepatocyte spheroids grown in Gri3D® or ULA 96WP plates (CellTiter-Glo® 3, Promega). D. Images depicting untreated and treated (1 µM ketoconazole) spheroids in Gri3D® or ULA 96WP plates. E. CYP3A4 inhibition upon treatment with Ketoconazole (P450-GloTM CYP3A4). Data are mean ± s.d. n = 3. Data from SUN bioscience in collaboration with Tecan, Promega, and Doppl SA.
Dr. Vural states that Gri3D® plates have significantly improved handling of spheroidal organ models in her lab. Unlike conventional plates where spheroids are easily lost during media exchange, Gri3D® plates allow for rapid and almost error-free handling, even for beginners. Additionally, the plates offer numerous technical replicates within a single well, providing flexibility ranging from analyzing soluble factors to performing (immuno)fluorescence staining3.
Thanks to these features, Dr. Vural’s team was able to create more efficient workflows, increasing throughput while saving time. Previously, processing spheroids for immunofluorescence staining and microscopy was challenging due to spheroid loss and varying focal planes. Gri3D® has mitigated these issues, allowing them to image spheroids more easily as they remain in the same focal plane.
Seamless integration with automation and imaging systems
Plate manipulation, including media exchange through the pipetting port, can be automated using liquid handling solutions. For example, researchers at the University of Edinburgh opted for the Viaflo (Integra) to automate Gri3D® for induced pluripotent stem cell-derived (iPSC) liver organoids generation5. In recent collaborative work, the SUN bioscience team could establish a liver spheroids automated workflow using Gri3D® plates on the Tecan Fluent® workstation2, reducing full plate media exchange time from three minutes (manual) to just 45 seconds.
Gri3D® microwell plates are designed according to SBS-recommended microplate specifications to integrate with imaging and analysis systems. SUN bioscience is actively collaborating with major life science equipment manufacturers to address applications that require custom solutions.
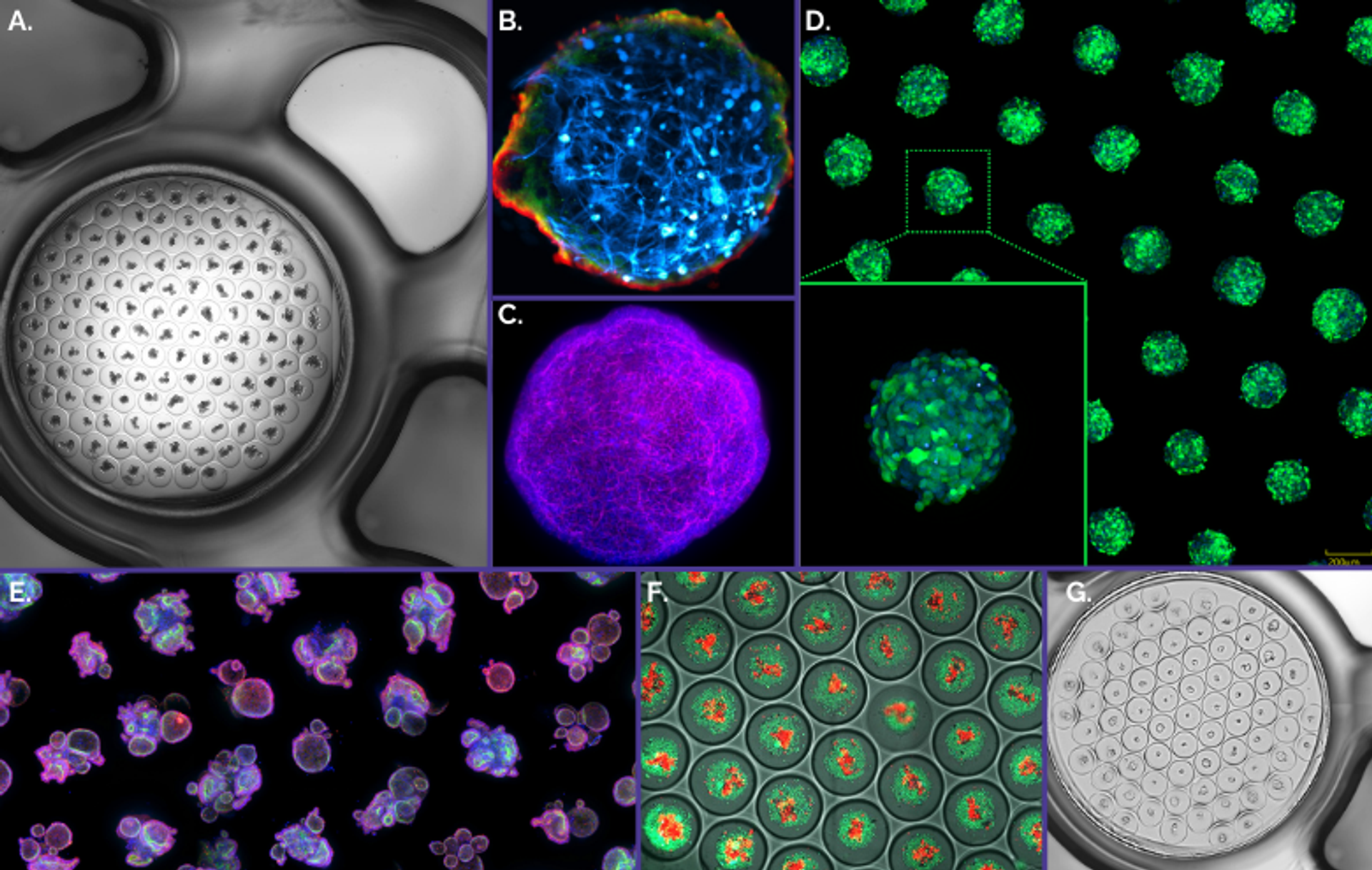
Figure 3. Gri3D® integrates with imaging equipment of major life science brands A. Mouse small intestinal organoids imaged with a Nikon Ti-E2. B. Blood-brain-barrier assembloids imaged with a Zeiss LSM700 confocal microscope4. C. Mouse liver organoid imaged with a Zeiss LSM710 confocal microscope. D. GFP-tagged HEK293 spheroids imaged with a Yokogawa CV8000 system. E. Human rectal organoids imaged on a Molecular Devices ImageXpress® Micro Confocal imaging system. F. Human colorectal cancer organoids co-cultured with TILs6 imaged with a GE Healthcare IN Cell Analyzer. G. Human small intestinal organoids imaged with a TECAN Spark® Cyto multimode plate reader.
Immuno-oncology assay for high-throughput tumoroid analysis
Researchers at the Federal Institute of Technology in Lausanne (EPFL) have developed an innovative immuno-oncology T-cell killing assay on Gri3D® to address the need for ex vivo models that can effectively assess immunotherapeutic treatments in a high-throughput and patient-specific manner6. This assay leverages patient-derived tumoroids with recreated immune microenvironments that are optimized for high-content evaluation of tumor-infiltrating lymphocyte (TIL) functionality.
In brief, colorectal cancer cells are first seeded into the 500-micrometer Gri3D® platform; where they form organoids over the course of a few days. These organoids are then co-cultured in direct contact with TILs, and the TIL infiltration into the organoids is subsequently analyzed. Previously, the team at EPFL faced challenges with uncontrolled organoid growth in Matrigel domes, resulting in “poor cell aggregation and low imaging resolution”. By utilizing Gri3D®, efficient cell aggregation occurs at predefined positions (the microwells) in a liquid matrix environment, resulting in “exceptionally homogeneous organoid formation”, as noted by the authors. They assert that this setup enhances cell–cell interactions within microcavities, supports reliable long-term tumoroid tracking, and allows for high-throughput, high-resolution imaging across multiple homogeneous replicates generated with minimal effort. Moreover, Gri3D® offers comparable results to standard Matrigel dome culture models, while providing superior resolution and throughput.
Gri3D® provides unprecedented opportunities to evaluate tumor immunogenicity, characterize responses to immunomodulators, and explore novel approaches for personalized immuno-oncology, advancing the ability to tailor treatments to individual patients' cellular and molecular profiles.
SUN bioscience’s microwell plates, with their high customization capabilities tailored to organoid workflows, are instrumental in advancing organoid research. Gri3D® plates are not only enhancing the development of new organoid models in academic research but also driving broader adoption of organoid technologies in the industry. As the field of organoid research evolves, SUN bioscience’s Gri3D® technology is poised to play a key role in accelerating assay development and expanding its applications.
Learn more and read what our customers think about SUN bioscience’s Gri3D® microwell plate catalog providing customizable consumables for precision, efficient, reproducible organoid workflows.
Request pricing for Gri3D® microwell platesReferences
1. Brandenberg, N., Hoehnel, S., Kuttler, F. et al.High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng 4, 863–874 (2020). https://doi.org/10.1038/s41551-020-0565-2. [online] Available at: https://www.nature.com/articles/s41551-020-0565-2
2. Oberdanner C., Clapés Cabrer M., Scaling drug response experiments in liver spheroids: Multiplexing 3D cell-based assays and imaging in a microwell platform. [online] Available at:https://www.selectscience.net/webinar/scaling-drug-response-experiments-in-liver-spheroids-multiplexing-3d-cell-based-assays-and-imaging-in-a-microwell-platform-2138
3. Heidari Khoei, H., Javali, A., Kagawa, H. et al.Generating human blastoids modeling blastocyst-stage embryos and implantation. Nat Protoc18, 1584–1620 (2023). https://doi.org/10.1038/s41596-023-00802-1. [online] Available at: https://www.nature.com/articles/s41596-023-00802-1
4. Simonneau, C., Duschmalé, M., Gavrilov, A. et al. Investigating receptor-mediated antibody transcytosis using blood–brain barrier organoid arrays. Fluids Barriers CNS 18, 43 (2021). https://doi.org/10.1186/s12987-021-00276-x. [online] Available at: https://fluidsbarrierscns.biomedcentral.com/articles/10.1186/s12987-021-00276-x
5. Lucendo-Villarin B., Meseguer-Ripolles J., Drew J., et al.Development of a cost-effective automated platform to produce human liver spheroids for basic and applied research. Biofabrication 13, 1 (2020). doi: 10.1088/1758-5090/abbdb2. [online] Available at: https://iopscience.iop.org/article/10.1088/1758-5090/abbdb2
6. Dutta, D., Lorenzo-Martín, L.F., Rivest, F. et al.Probing the killing potency of tumor-infiltrating lymphocytes on microarrayed colorectal cancer tumoroids. npj Precis. Onc. 8, 179 (2024). https://doi.org/10.1038/s41698-024-00661-3. [online] Available at: https://www.nature.com/articles/s41698-024-00661-3

