Product NewsDrug discovery > Pre-Clinical Development
Safety testing in immune humanized platforms
8 Apr 2024
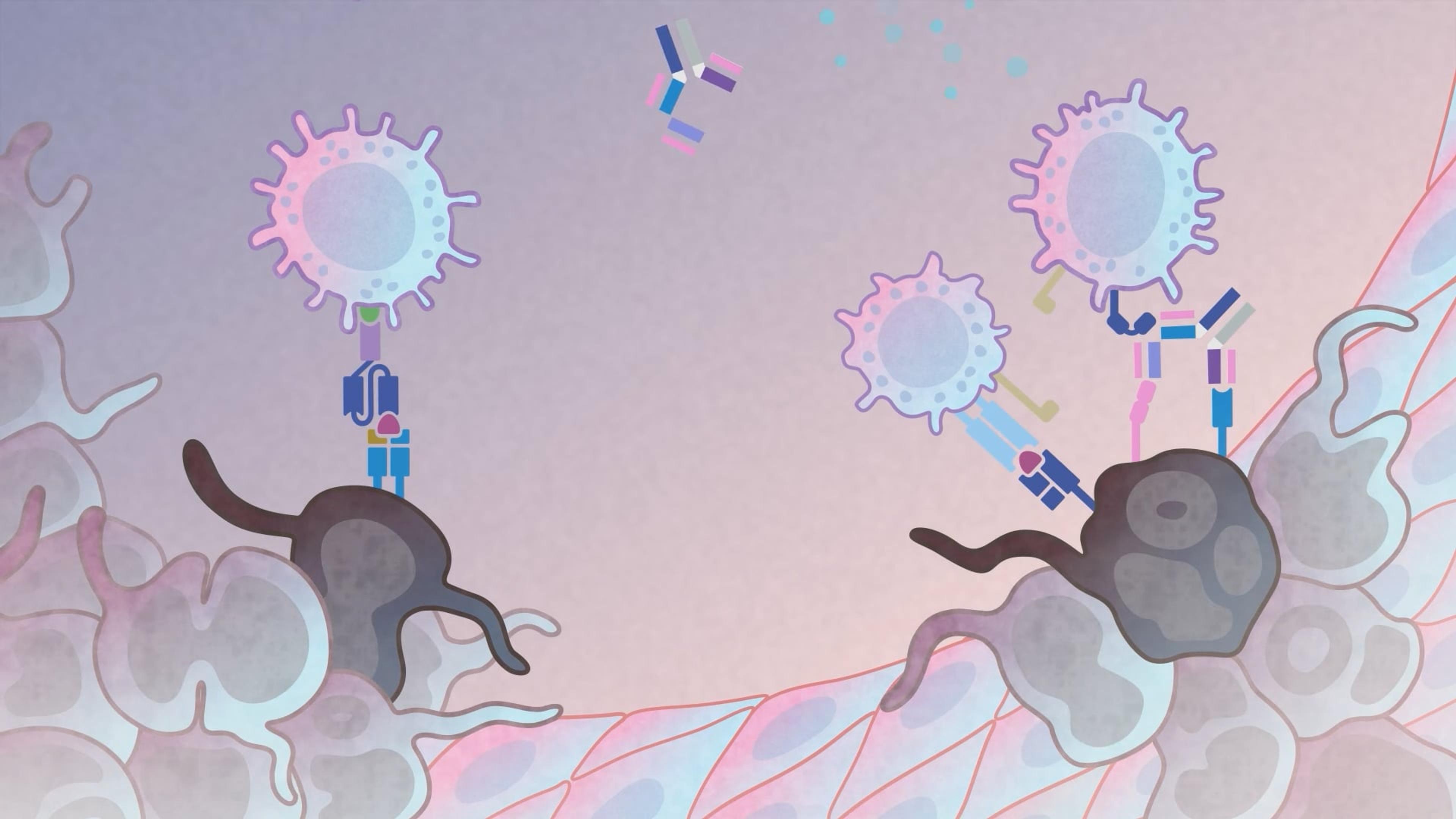
Immune humanized platforms, particularly utilizing highly immunodeficient mice, facilitate co-engraftment of human immune cells and tumors, enabling the evaluation of therapies targeting human-specific biological responses. These platforms efficiently incorporate multiple donors or tumor samples to model and predict human variation in response, and systemic consequences of toxic cytokine release for safety assessment. Dr. Brian Soper from The Jackson Laboratory presents preclinical data covering monoclonal antibody-based human immune modulation, donor-specific differences in bispecific antibody-mediated tumor efficacy and cytokine release, and CAR T cell-mediated tumor efficacy and safety.