Search results for "Rigaku Analytical Devices"
Selected Filters:
Precision and price down to a point!
Resolian acquires China-based bioanalytical CRO Denali Medpharma
Resolian can now initiate projects on four continents and easily transfer validated methods across labs
The NEW PURELAB® Pharma Compliance 2022
Simplifying scientific discovery: A new liquid handling solution with ease-of-use at its heart
Discover the unique features of an innovative new pipette controller and gain top tips for getting the most out of your apparatus
Inscopix nVue System
Bruker Fluorescence MicroscopyInscopix’s nVue system is a complete miniscope platform able to register dual-color calcium signals from two cellular populations simultaneously with single-cell resolution in freely behaving animals. A streamlined workflow supports static and dynamic cell imaging, blood flow imaging, or genetically encoded optical neurotransmitter sensors, all within the same field of view. Furthermore, this technology is supported by multim…
Metrohm USA Debuts a New Look and New Products
Versatile low-light barcode reader
Semi automatic microplate sealer
Effective Automatic Sorting of Valuable Samples
Sarstedt ELISA Plates in Black and White
Ensuring safe drinking water
Faster Flow Rates for Sterile Filtration
ELISA Workflow Using EMax Plus Microplate Reader & MultiWash+ Microplate Washer
Success for Virtual Summit on Cancer and Immunology Research: Second SelectScience Virtual Summit reaches over 780,000 scientists
Science industry comes together online to share knowledge and provide technology solutions for cancer and immunology research
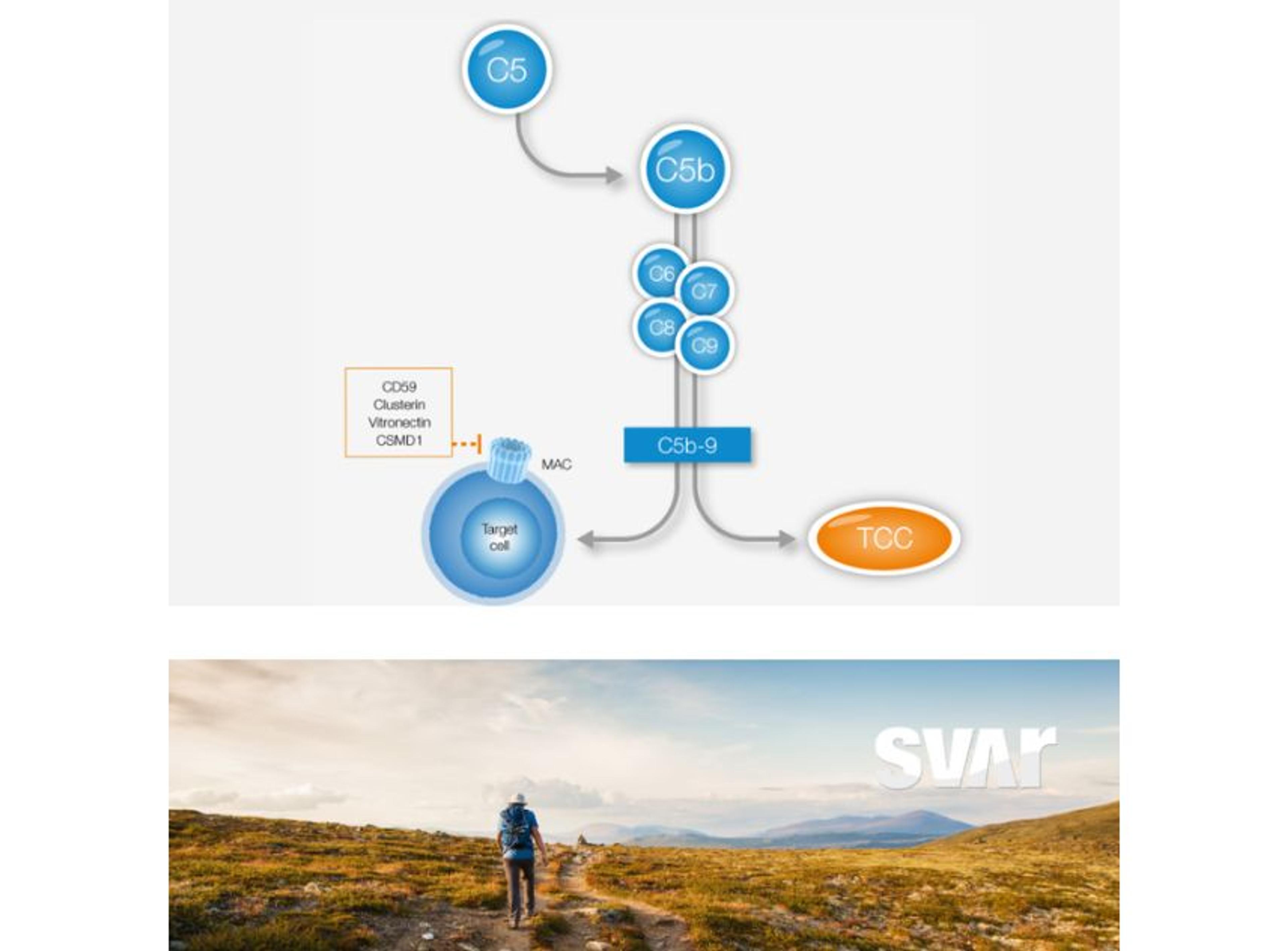
Complement TCC
Svar Life Science ABThe Complement TCC is a flexible and easy to use immunoassay for determination of the terminal complement complex (TCC, also known as sC5b-9) in human plasma.