Drug discovery > Clinical Development Products & Reviews
Products, services, reviews and techniques used in lead molecule testing in humans through clinical trials and regulatory services, and data analysis.
Selected Filters:
Unity Real-Time LT
Bio-RadAn entry-level desktop QC data management software providing basic QC rules, charts, and peer reports
Unity Real-Time Online
Bio-RadAdvanced online QC data management software to facilitate regulatory compliance under CLIA and ISO 15189 and provides advanced charts, reports, data analysis, and comprehensive audit trails.
Unity Real-Time
Bio-RadAdvanced desktop QC data management software for expert users to facilitate regulatory compliance under CLIA and ISO 15189 and provides advanced charts, reports, data analysis, and comprehensive audit trails.
CBS Nanny 24/96/384-Well Kits Flights
Cellbox SolutionsThe complete leak proof and gas permeable packaging solution for culturing and shipping in 24/96/384 multiwell plate formats.
CBS Breathy Kits Flight
Cellbox SolutionsLeak proof and gas permeable secondary packaging material available in two sizes to combine with your own cell culture vessels.
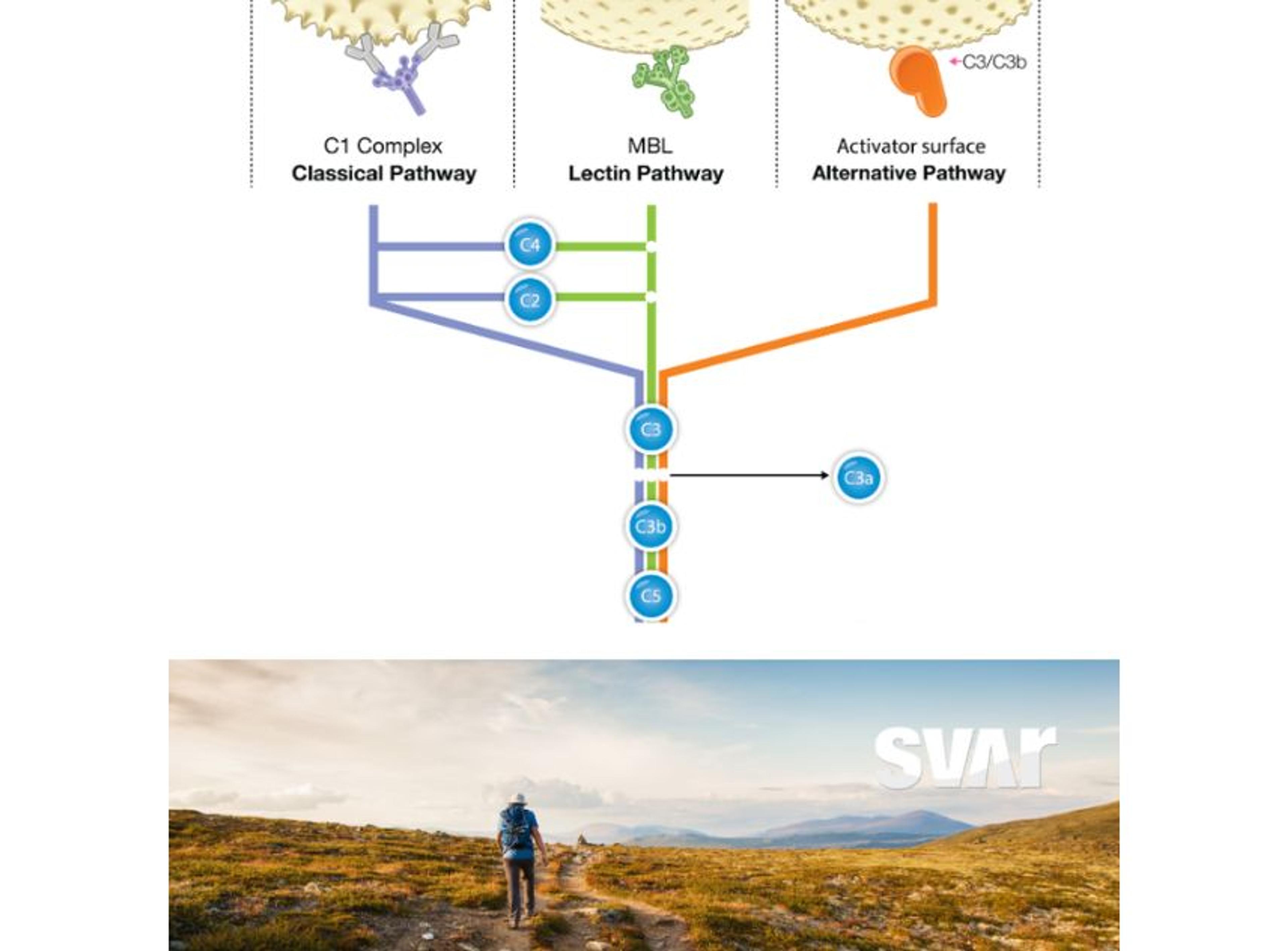
Wieslab® Functional Complement Assays
Svar Life Science ABThe Wieslab® Complement Pathway-specific Functional assays are well known within the complement world, used both as an alternative to hemolytic assays and a diagnostic tool for complement functional assessment. Based on the ELISA setup, the assays provide a robust and standardized assay platform, delivering fast, reliable, and easy-to-interpret results within 3 hours.
Micronic Rack Reader DT520
MicronicThe high speed Micronic Rack Reader DT520 is designed to quickly read single tubes and rack barcodes in one ergonomic device. Readout time is less than one second.
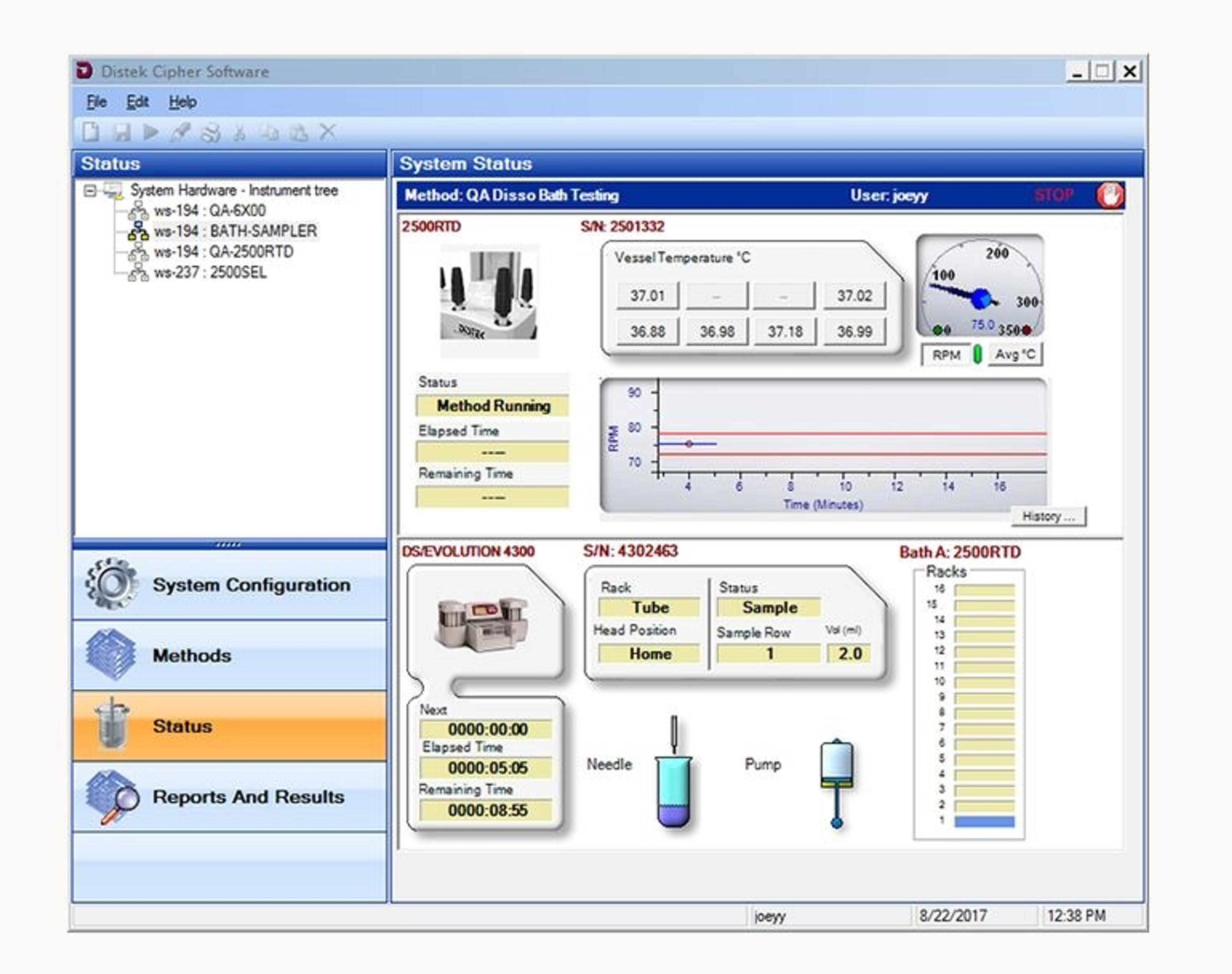
Cipher Dissolution Instrument Control Software
Distek, Inc.The Cipher dissolution control software is a Windows-based application designed and developed by Distek which can be installed locally or networked providing full access to any Distek dissolution instrument on the network. Cipher offers the flexibility to set the level of security appropriate for your laboratory and will meet 21 CFR part 11 compliance.
PURedit™ Protein
MerckRecombinant PURedit Cas9 protein from Streptococcus pyogenes is a ready-to-use reagent for genome engineering experiments
Caco-2 Cells
MerckEnable assessment of drug transport by comparison between the wild-type (WT) and knockout cell lines
Ultra High Throughput Colloidal Gold Card Assembly System MRA-LSF-840
MEGAROBO TechnologiesMegarobo's new MRA-LSF-840 series ultra-high-throughput kit assembly system can meet the needs of reagent card assembly in terms of throughput, compatibility, and yield. At the same time, it is equipped with card loading and packaging modules, and each module can be operated independently or combined for production.
High Throughput Reagent Filling System MRA-LSF-850
MEGAROBO TechnologiesEfficient: Up to 8000-10000 pcs/h Flexible: Compatible with multiple types of tubes such as disposable virus sampling tubes Intuitive: Visualization software & touch screen Modular: Including laminar flow, labeling and coding, and CCD modules, allows for personalization Stable: Stable operation, high yield rate Effortless: Only 1 -2 people are needed to complete the whole process
High Throughput Reagent Filling System MRA-LSF-860
MEGAROBO TechnologiesEfficient: Up to 8000-10000 pcs/h Flexible: Compatible with multiple types of tubes such as disposable virus sampling tubes Intuitive: Visualization software & touch screen Modular: Including laminar flow, labeling and coding, and CCD modules, allows for personalization Stable: Stable operation, high yield rate Effortless: Only 1 -2 people are needed to complete the whole process
1.40ml Internal Thread Screw Cap Tubes
NBS ScientificThe Micronic 1.40ml internally threaded screw cap tubes are available with a V- or U-shaped interior bottom.
Gap Analysis Tool
Clinical and Laboratory Standards InstituteLearn how to perform a gap analysis of laboratory quality activities to help your laboratory meet minimum QMS requirements.
QPS Global Regulatory Affairs Services
QPSQPS Global Custom-Built Regulatory Services Can Transform Clinical Trials. QPS Global Regulatory Affairs (GRA) service offerings focus on helping pharmaceutical, biotechnology, and medical device companies to develop custom-built research solutions that forge expedited regulatory pathways from discovery to global commercialization and onwards through product lifecycle support.
QPS Clinical Phase I/IIa Development Services
QPSMoving quickly and safely through Phase I/IIa trials is critical to successful drug development. The QPS Phase I research facilities feature more than 550 beds across six strategically located facilities on three continents. QPS is well known for its success in first-in-human clinical trials. All Phase I sites are staffed by expert clinical pharmacology teams that routinely conduct hundreds of phase I/IIa studies annually.
QPS Clinical Phase II/IV Development Services
QPSAt QPS, we realize that in today’s late stage drug development space you face many challenges. With our deep experience and broad global presence, QPS is in an excellent position to offer solutions to all of the above needs. With its site management & monitoring teams operating from 30 locations on three continents (Asia/Pacific, USA, and Europe), QPS has become a new strong player in Phase II/IV clinical research services.
CLARION™
bioMérieux USAOptimize efficiency and outcomes with data-driven infectious disease management.
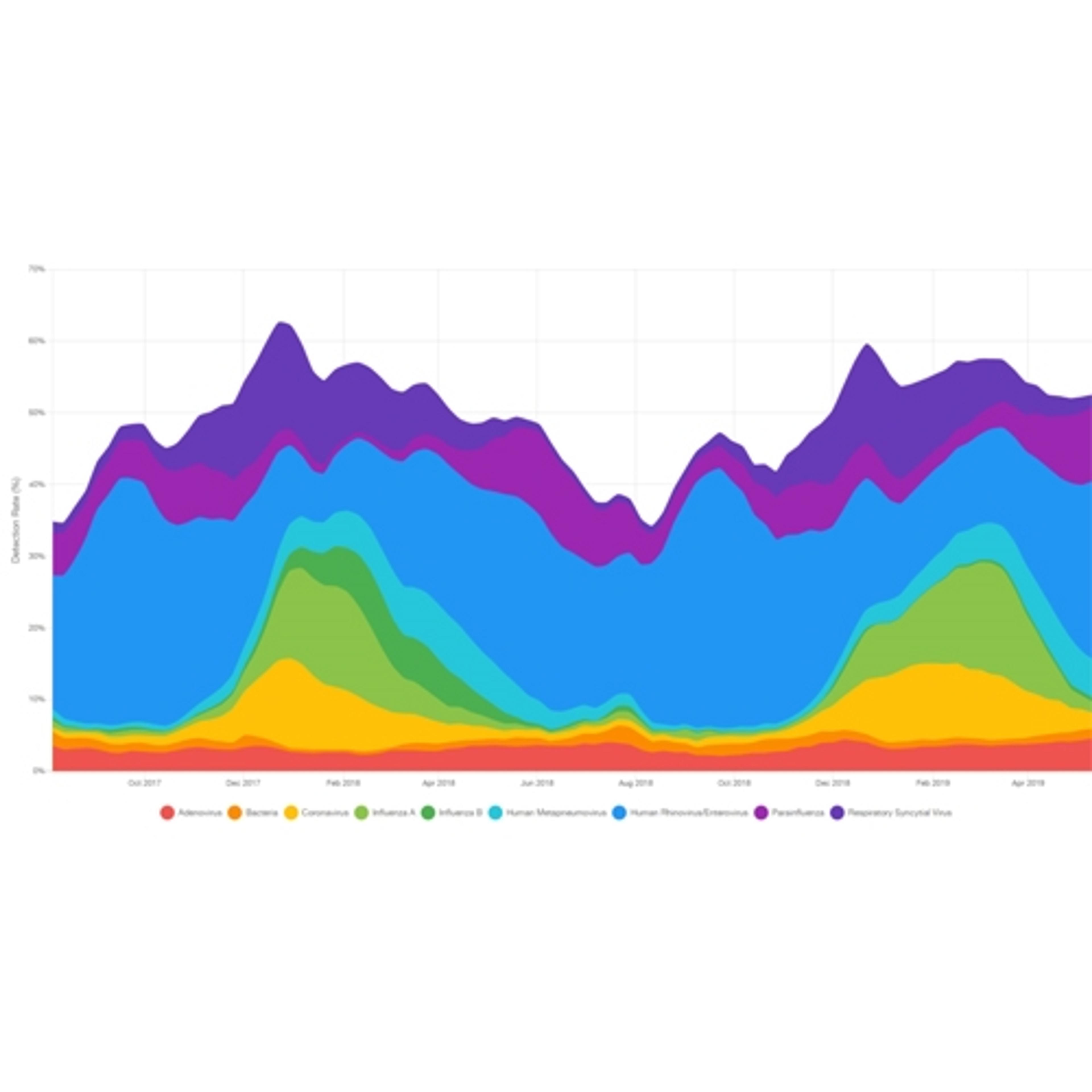
BIOFIRE® Syndromic Trends
bioMérieux USAVisual insights into pathogen circulation trends in near real time.