BIOFIRE® Respiratory 2.1 (RP2.1) Panel
The BIOFIRE RP2.1 Panel tests for 22 of the most likely respiratory targets, including viruses and bacteria, with results in about 45 minutes.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Great result
Respiratory pathogens
Easy fast and efficient
Review Date: 27 Jun 2023 | bioMérieux USA
Every lab should have this test offered!
Analysis of nasopharyngeal swab for the detection of relevant respiratory tract pathogen.
The Biofire Respiratory Panel 2.1 is a one stop test for all the relevant respiratory tract pathogen. It is quick, easy to use, and accurate!
Review Date: 17 Apr 2023 | bioMérieux USA
Easy setup and fast TAT!
Patient testing in hospital environment.
The BioFire Filmarray products are easy to use and each kit is standardized. If you know how to set up one type you pretty much know how to set up most of them. I do wish the kits came preassembled in easy grab and go pouches with one of each reagent and such in them instead of separated in big bags. Repeated opening/closing of the bags and hands in and out increases the risk of contamination. So, packaging could be simplified and streamlined, but otherwise we are very happy with the products.
Review Date: 17 Apr 2023 | bioMérieux USA
Quality and speed make this an invaluable test offering for our patients and doctors.
Molecular diagnostics
The respiratory panel 2 enables us to detect various pathogens aside from SARS-cov-2 in cases of pneumonia, to quickly identify treatable etiology / agents. After -sales service is prompt and efficient. Although it costs more, it is worth it due to rapid results enabling timely life-saving intervention by clinicians.
Review Date: 15 Jun 2021 | bioMérieux USA
Great results within a couple of hours.
Respiratory infection diagnosis
The product provides easy and quick test for common respiratory microorganisms. It does not need to wait for multiple samples to run together so that TNT of the assay is much reduced.
Review Date: 15 Jun 2021 | bioMérieux USA
Effective and conclusive diagnosis can't be achieved without this testing kit.
Diagnosis
It gives a wide range of diagnosis for viruses and bacteria.
Review Date: 15 Jun 2021 | bioMérieux USA
Excellent machine
Molecular
Very good
Review Date: 15 Jun 2021 | bioMérieux USA
Great!
Covid
Excellent product. Very useful for ER patients with respiratory profile.
Review Date: 6 Jun 2021 | bioMérieux USA
With Biofire, no need to guess, just know.
Syndromic testing for respiratory patients
It helps to accelerate the workflow to get a quick answer in order to help the patient, all we need is the sample without any preparation. Takes 45 mins to get an accurate and comprehensive result. Since respiratory symptoms are often the same we need such a panel to reduce the patient length of stay and reduce the number of tests needed. Also the fast response from the Biofire team for any concerns is an advantage.
Review Date: 25 May 2021 | bioMérieux USA
Excellent instrument and product. Zero communication with users.
Molecular microbiology
Very bad communication between account manager and clients.
Review Date: 8 Feb 2021 | bioMérieux USA
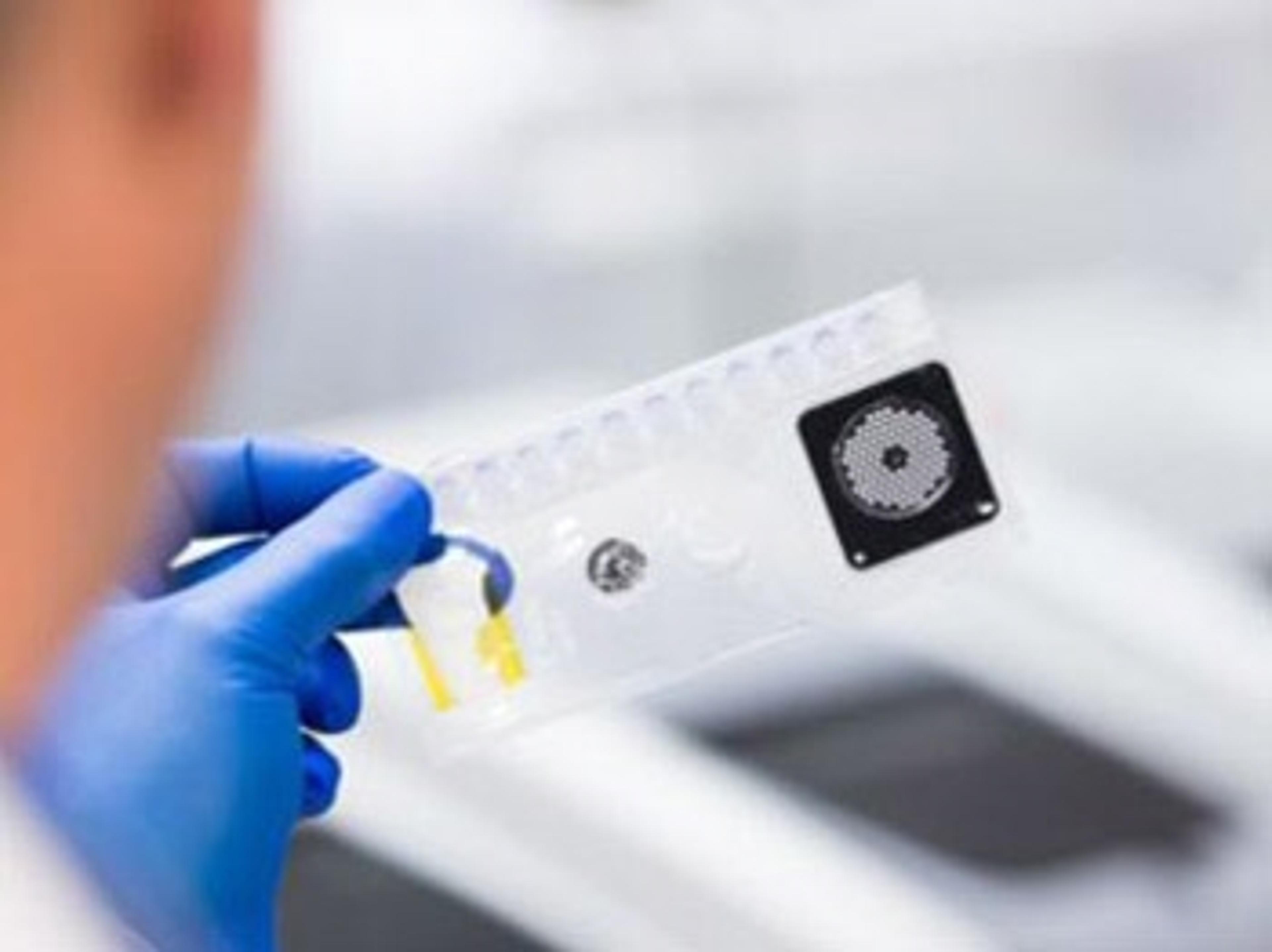
The BIOFIRE RP2.1 Panel helps clinicians quickly diagnose respiratory infections, which present with nearly indistinguishable symptoms. Respiratory infections can be caused by many viruses and bacteria—not just SARS-CoV-2 or influenza. The BIOFIRE RP2.1 Panel uses a molecular approach to accurately detect and identify the pathogens most commonly associated with respiratory infections.
- Detects and identifies 18 viral targets, including SARS-CoV-2, and 4 bacterial targets
- Results are available in about 45 minutes
- Available for use on the BIOFIRE® FILMARRAY® TORCH and the BIOFIRE® FILMARRAY® 2.0 systems