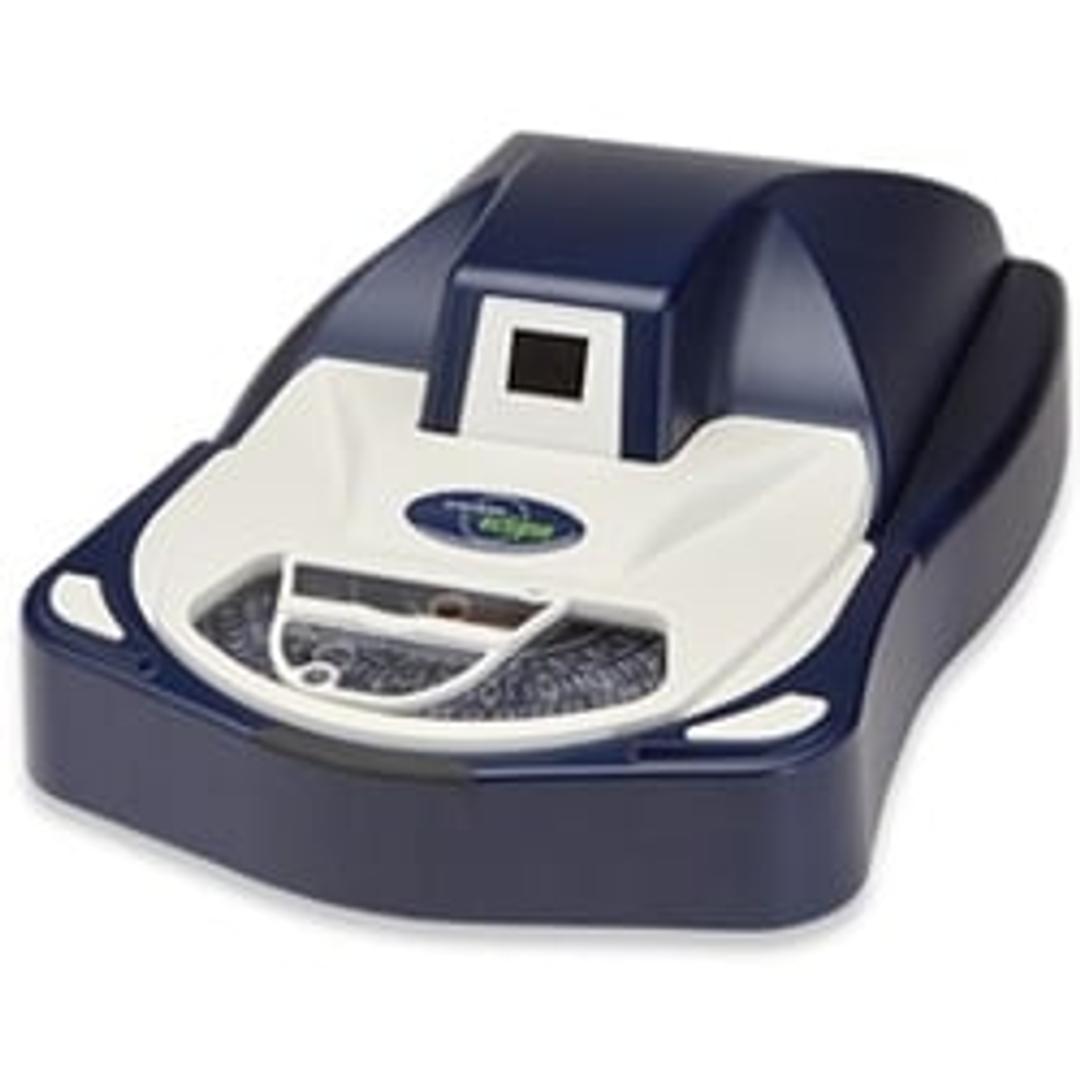
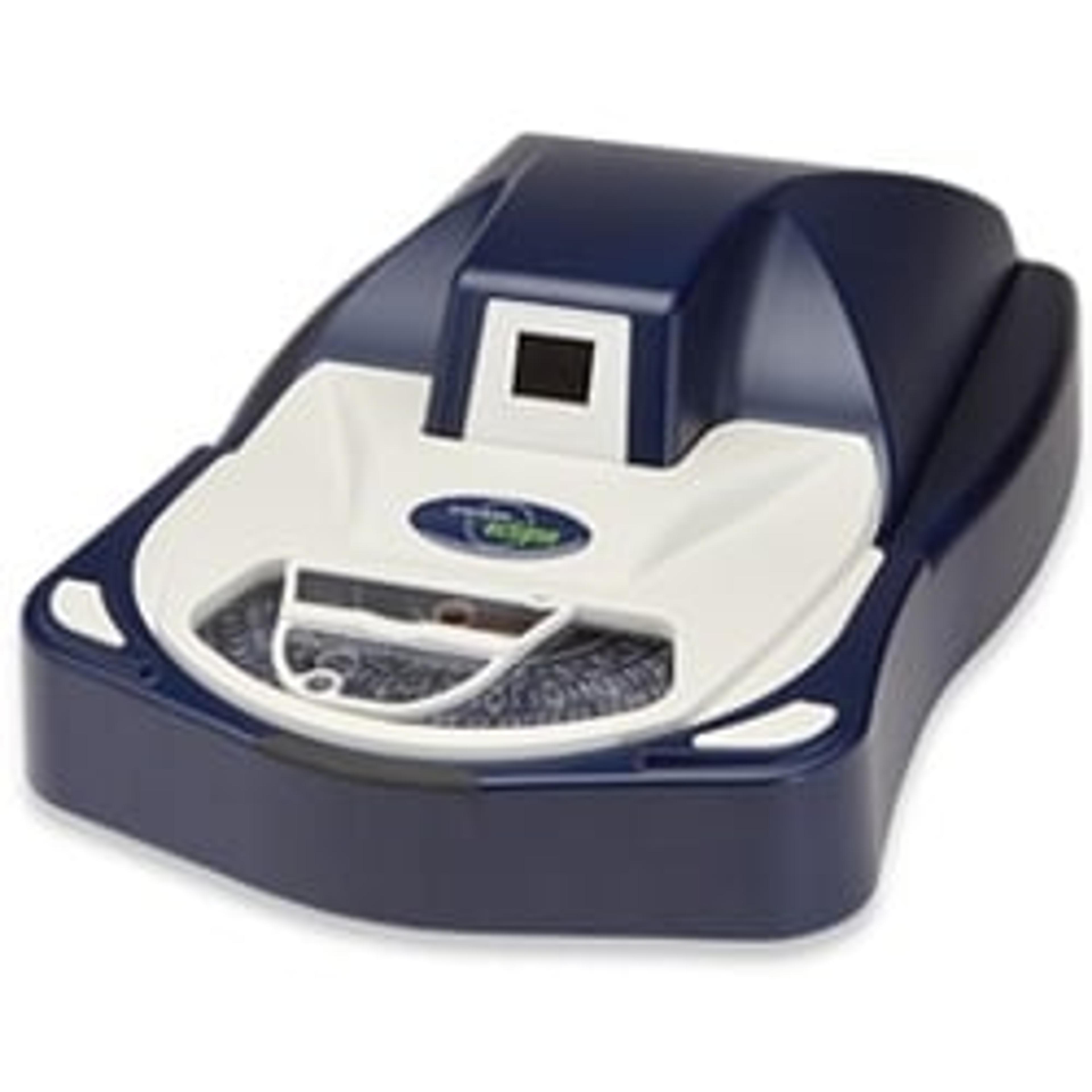
Sievers Eclipse Bacterial Endotoxins Testing (BET) Platform
The innovative Sievers Eclipse platform decreases assay setup time by up to 85% and reduces Limulus Amebocyte Lysate (LAL) reagent use by up to 90% while meeting all requirements of the harmonized pharmacopoeia: USP <85>, EP 2.6.14 and JP 4.01.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Fully compliant, 21-sample assay setup in as little as 9 minutes!
Testing endotoxins
Sievers Eclipse Bacterial Endotoxins Testing (BET) Platform is very good and innovative.
Review Date: 7 Apr 2021 | SUEZ
The innovative Sievers Eclipse platform decreases assay setup time by up to 85% and reduces Limulus Amebocyte Lysate (LAL) reagent use by up to 90% while meeting all requirements of the harmonized pharmacopoeia: USP <85>, EP 2.6.14 and JP 4.01. Through groundbreaking technology, the Eclipse platform significantly decreases pipetting steps, reduces operator-to-operator variability, and simplifies assay setup. The Eclipse platform leverages precise microfluidic liquid handling and embedded endotoxin to automate kinetic chromogenic assays. Throughput of 21 samples per plate is maintained without the complexity of robotics or the time and technique demands of a traditional assay.
Eclipse Platform Lab Features & Benefits
- Enables fully compliant, 21-sample assay setup in as little as 9 minutes
- Reduces Limulus Amebocyte Lysate (LAL) reagent use by up to 90%
- Meets all requirements of the harmonized pharmacopoeia: USP <85>, EP 2.6.14 and JP 4.01
- Embedded endotoxin for a standard curve and PPCs
- Provides segments for standard curves, samples, and PPCs
- Sensitivity down to 0.005 EU/mL
- Enterprise software solution that complies with 21 CFR Part 11 and Data Integrity guidelines
- Decreases the need for extensive operator training
- Reduces the risk of repetitive stress injury
- Increases daily sample throughput
- Uses commercially available, FDA licensed LAL
- Controls reaction temperature within 37 +/- 1 °C using incubating absorbance analyzer