Products & ReviewClinical Diagnostics
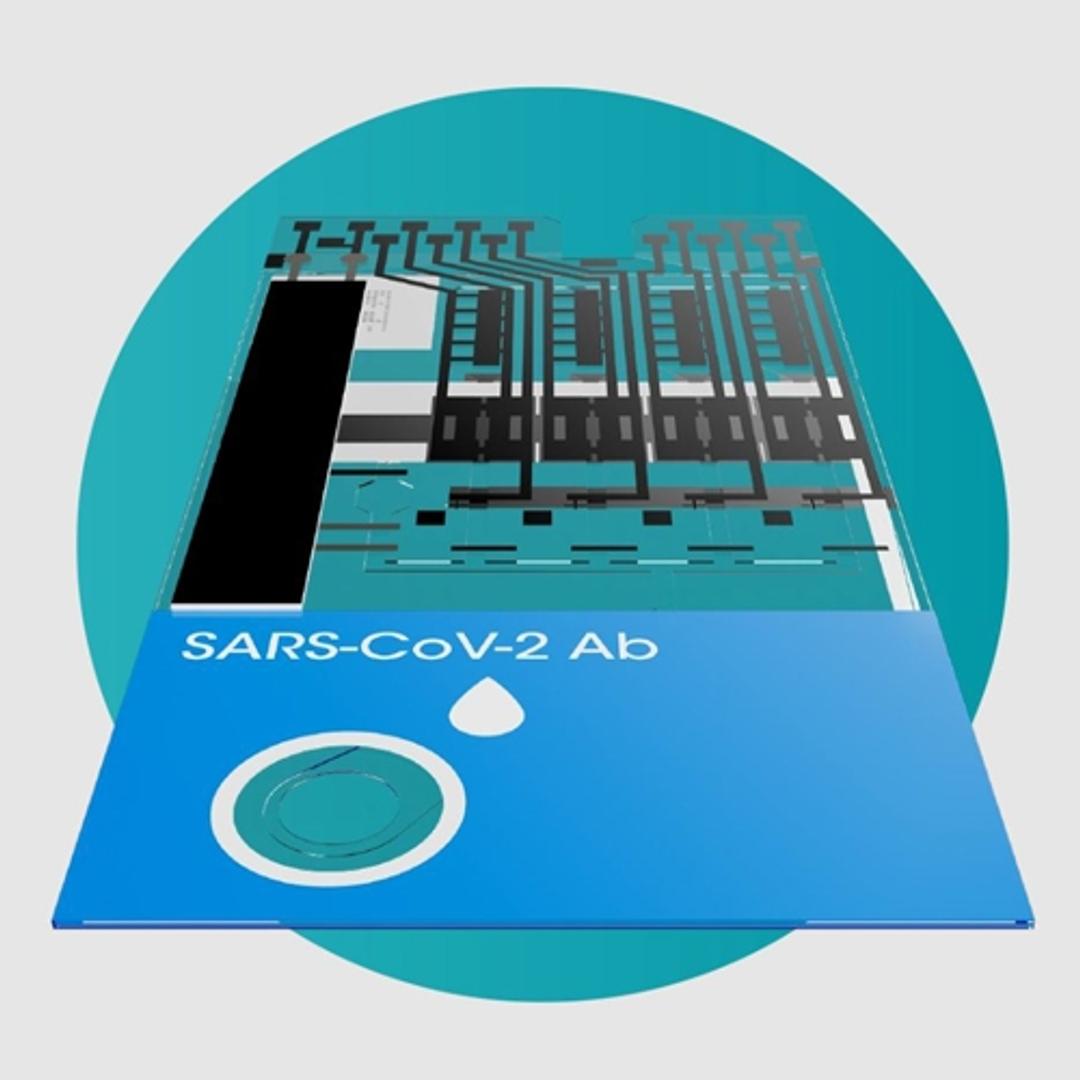
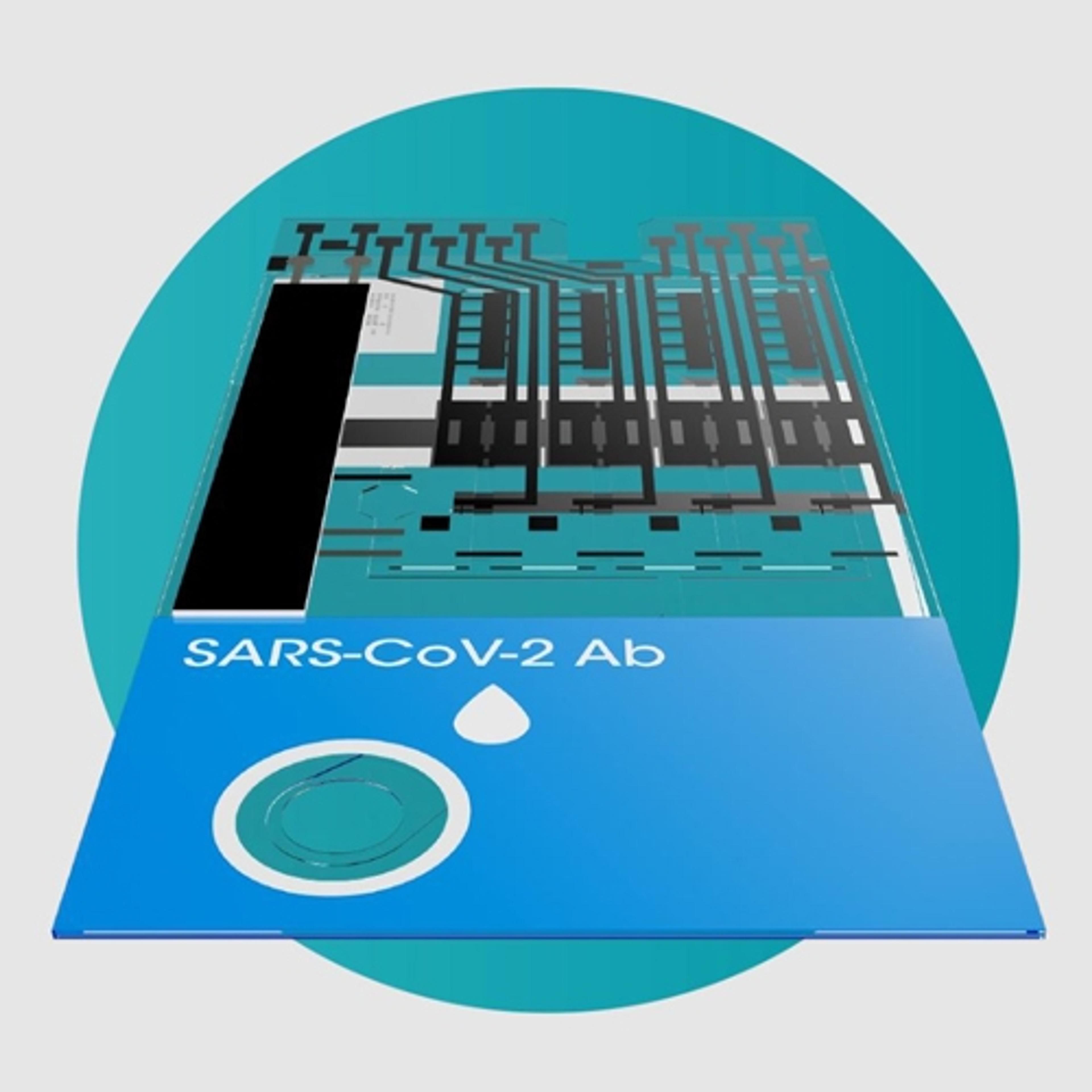
SARS-CoV-2 Ab Test
The LumiraDx SARS-CoV-2 Ab Test is a microfluidic immunofluorescence assay for qualitative detection of total antibodies to SARS-CoV-2 in human whole blood (capillary fingerstick or venous), plasma or serum for indication of recent or prior infection. Used with the LumiraDx Platform the Test delivers rapid results at the point of care.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Test benefits
The LumiraDx SARS-CoV-2 Ab Test is designed to be used in community care settings to identify individuals with an adaptive immune response to COVID-19, indicating recent or prior infection.
- Easy to implement in point of care settings
- Clinical agreement (direct fingerstick) in samples collected more than 8 days post RT-PCR
- 100% (62/62) positive agreement
- 100% (54/54) negative agreement
- Time to result in 11 minutes