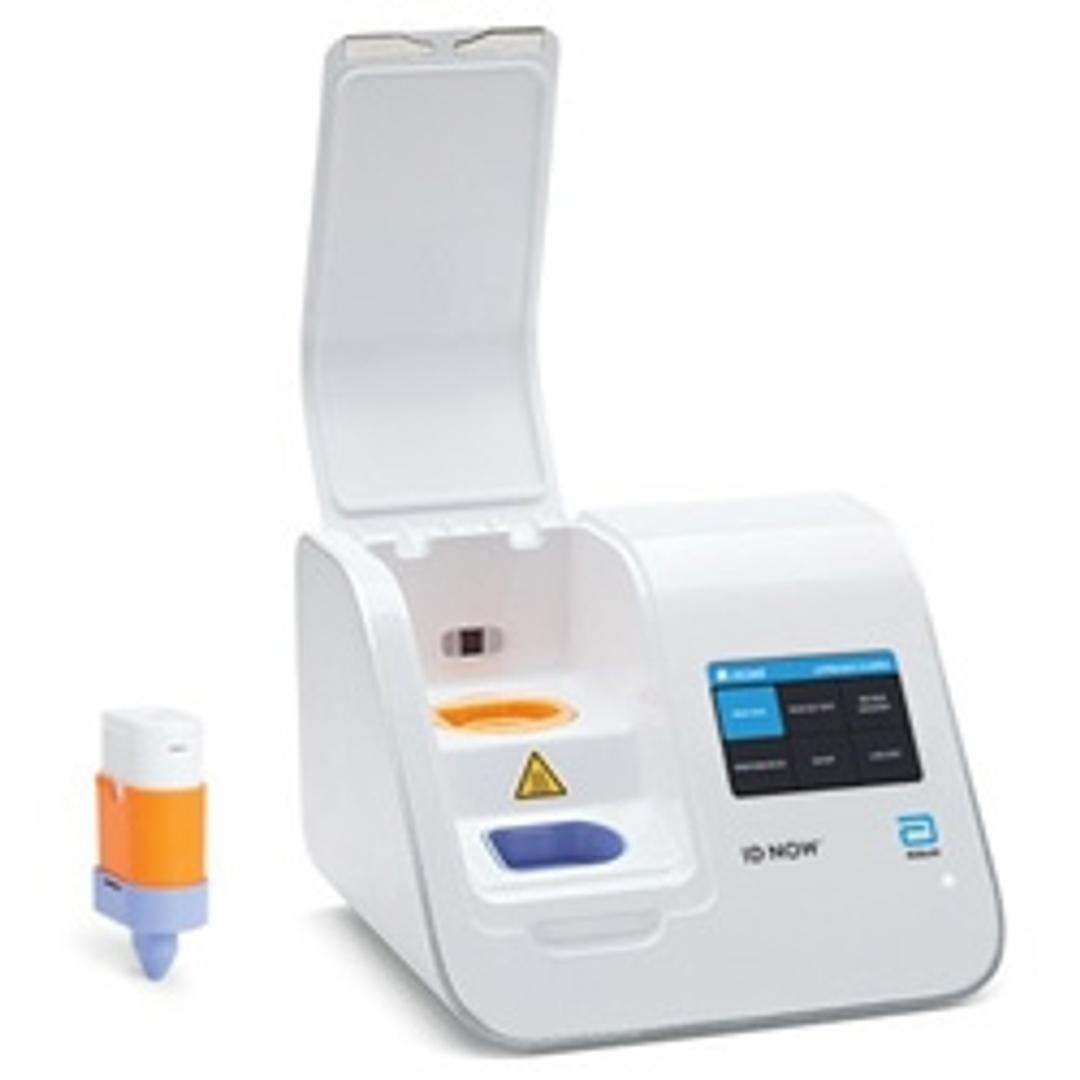
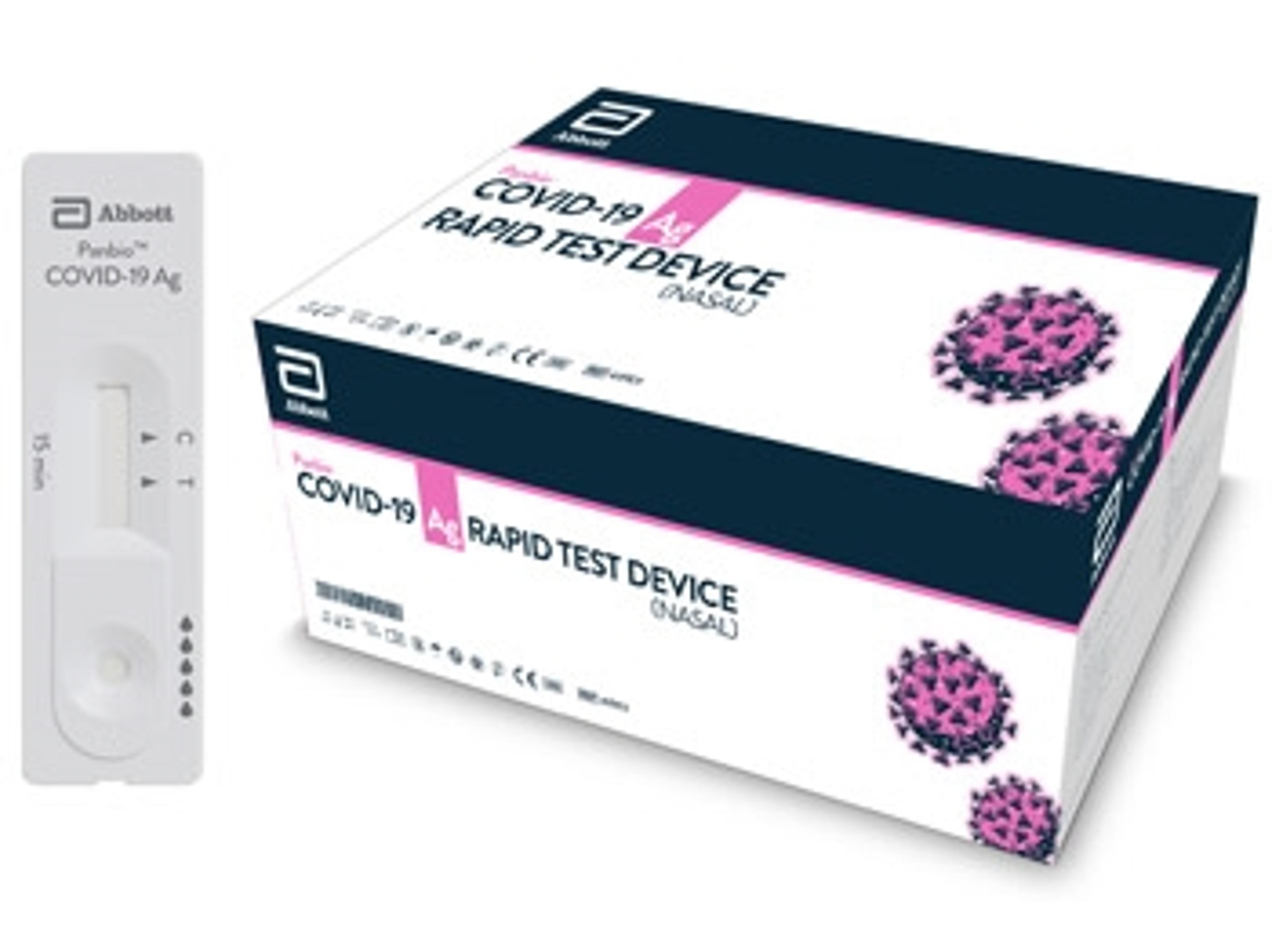
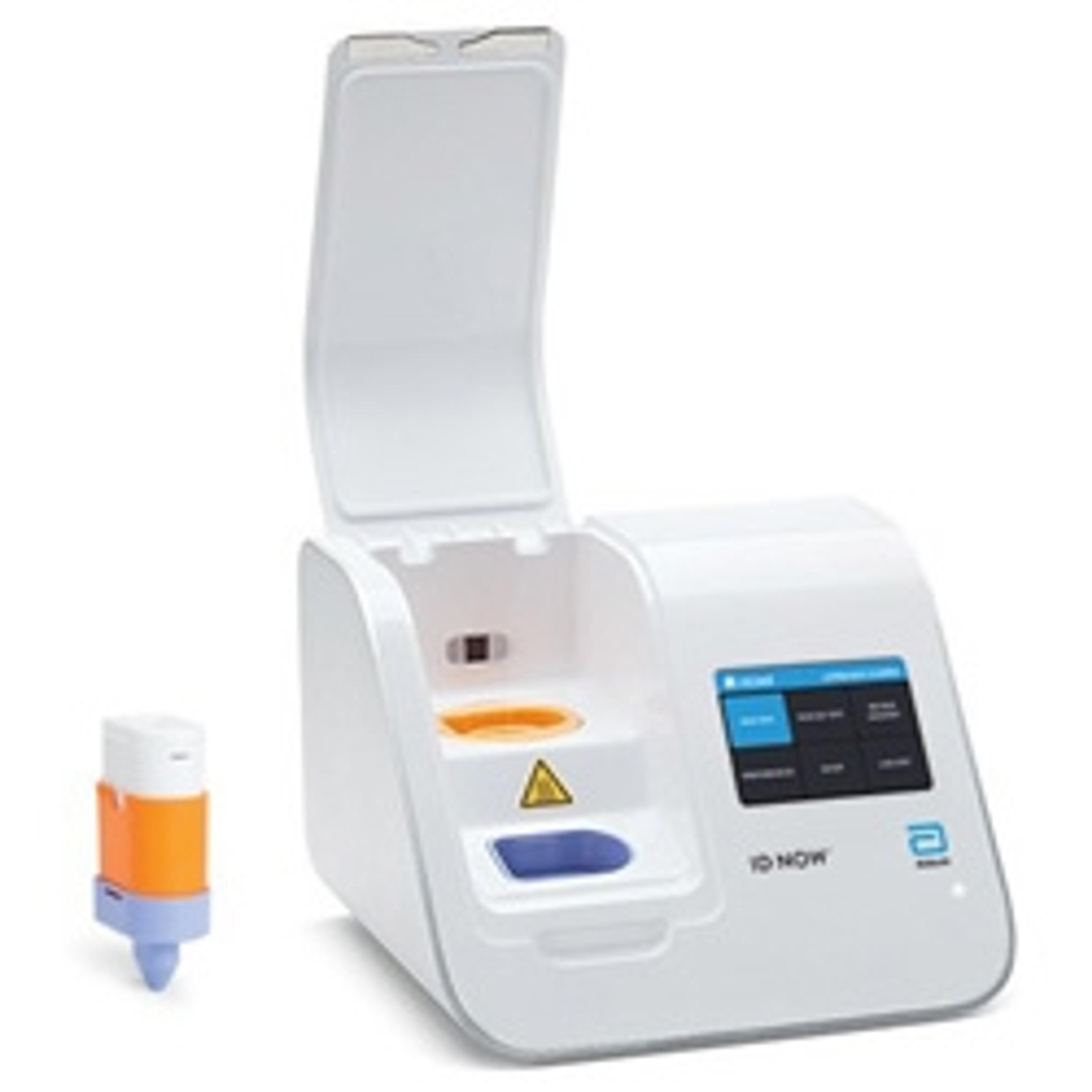
ID NOW™ COVID-19
Molecular. In minutes. On the front line.
Great instrument for out patient services and point of care needs.
COVID-19 PCR testing
Instrument usage is very easy. Assay limitations are greatly dependent on the collector. Appropriately collected sample will yield accurate results. False negativity rates increase with poor collection techniques. Assay is not as specific as other PCR methods however it's a step up from antigen testing.
Review Date: 23 May 2022 | Abbott
Great, easy and fast. reliable and accurate results. Ideal for rapid molecular test
COVID test
Easy-to-use equipment with step-by-step on-screen directions. Easy calibration and reliable results (compared to PCR). Elegant design and easy maintenance.
Review Date: 25 Mar 2022 | Abbott
Useful analyzer
Covid-19
Easy to use, and test is completed at the point of care. Results in 15 minutes. Other test can be run on the instrument.
Review Date: 19 Nov 2021 | Abbott
Very essential during this pandemic and supply chain issues.
Testing samples for COVID-19
This instrument has been a life saver for us as an additional platform for COVID testing. With supply shortages everywhere, we have come to rely on the consistent supply of COVID testing kits we have received. We have three of these units in our lab and they are well used with no issues. We have had very few invalid results and if we have an invalid times two on a sample, we run it on a different platform. QC is included with the kit so there is no extra expense.
Review Date: 15 Nov 2021 | Abbott
Lifesaver for our small hospital lab.
Testing samples for COVID-19
These little analyzers have been a saving grace for us during this pandemic. They are fast, easy to use, accurate and supplies are fairly easy to get.
Review Date: 24 Sept 2021 | Abbott
Always saving the day!
Diagnostic
We are able to perform point of care COVID19 test in less than 20 minutes helping with our logistics.
Review Date: 9 Feb 2021 | Abbott
The ID NOW™ COVID-19 assay is now available for use on the ID NOW platform under U.S. Food and Drug Administration Emergency Use Authorization (EUA). The ID NOW™ COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes, targeting the coronavirus (COVID-19) RdRp Gene.
Timely results enable healthcare professionals to make appropriate and more efficient treatment and infection control decisions. EUA supports flexible near-patient testing environments.
Benefits:
- Positive results may be detected in as little as 5 minutes
- Negative results in 13 minutes
- Molecular technology targeting COVID-19 RdRp gene
- Designed for near-patient testing in a variety of healthcare environments
- Room temperature storage
- Direct sample types include: Nasal, Throat, and Nasopharyngeal swabs
- Facilitates effective patient management