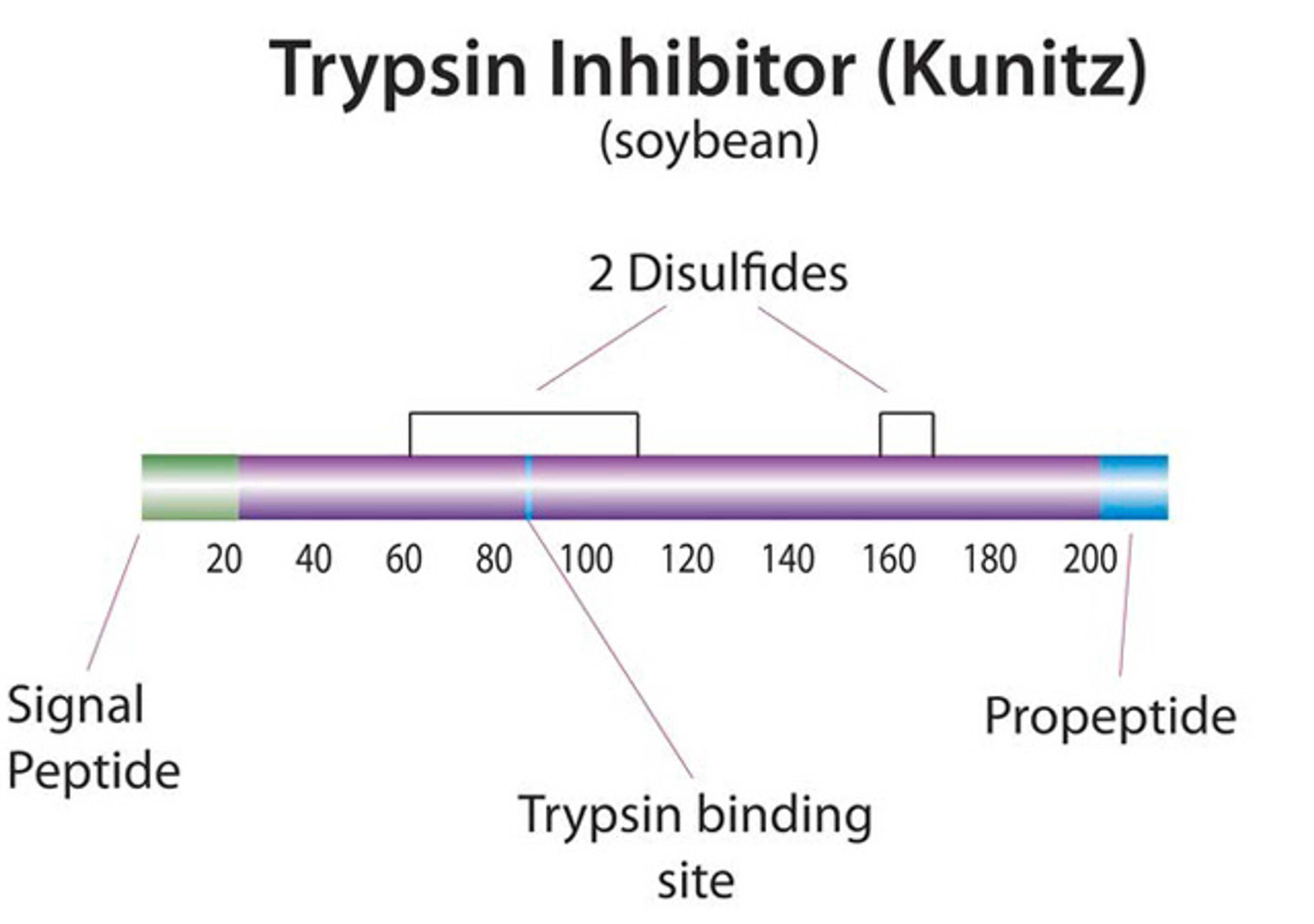
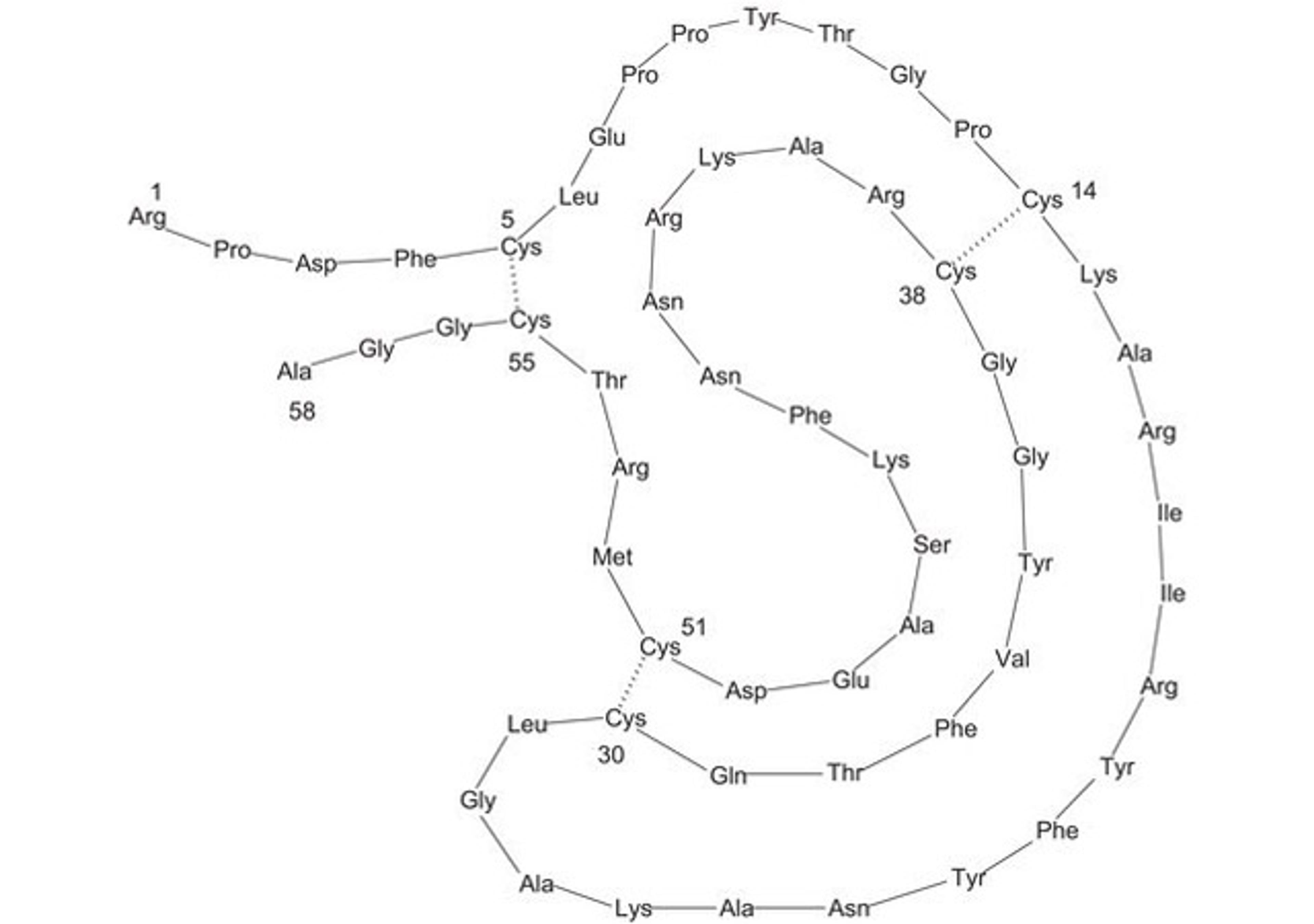
Products & ReviewClinical Diagnostics
EP43, Implementing a Laboratory Test Under Emergency Use Conditions
This white paper covers public health emergencies in general, the ways in which emergency use authorizations (EUAs) may be used, and the ramifications of EUAs for medical laboratories and other testing sites.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
This white paper covers public health emergencies in general, the ways in which emergency use authorizations (EUAs) may be used, and the ramifications of EUAs for medical laboratories and other testing sites.