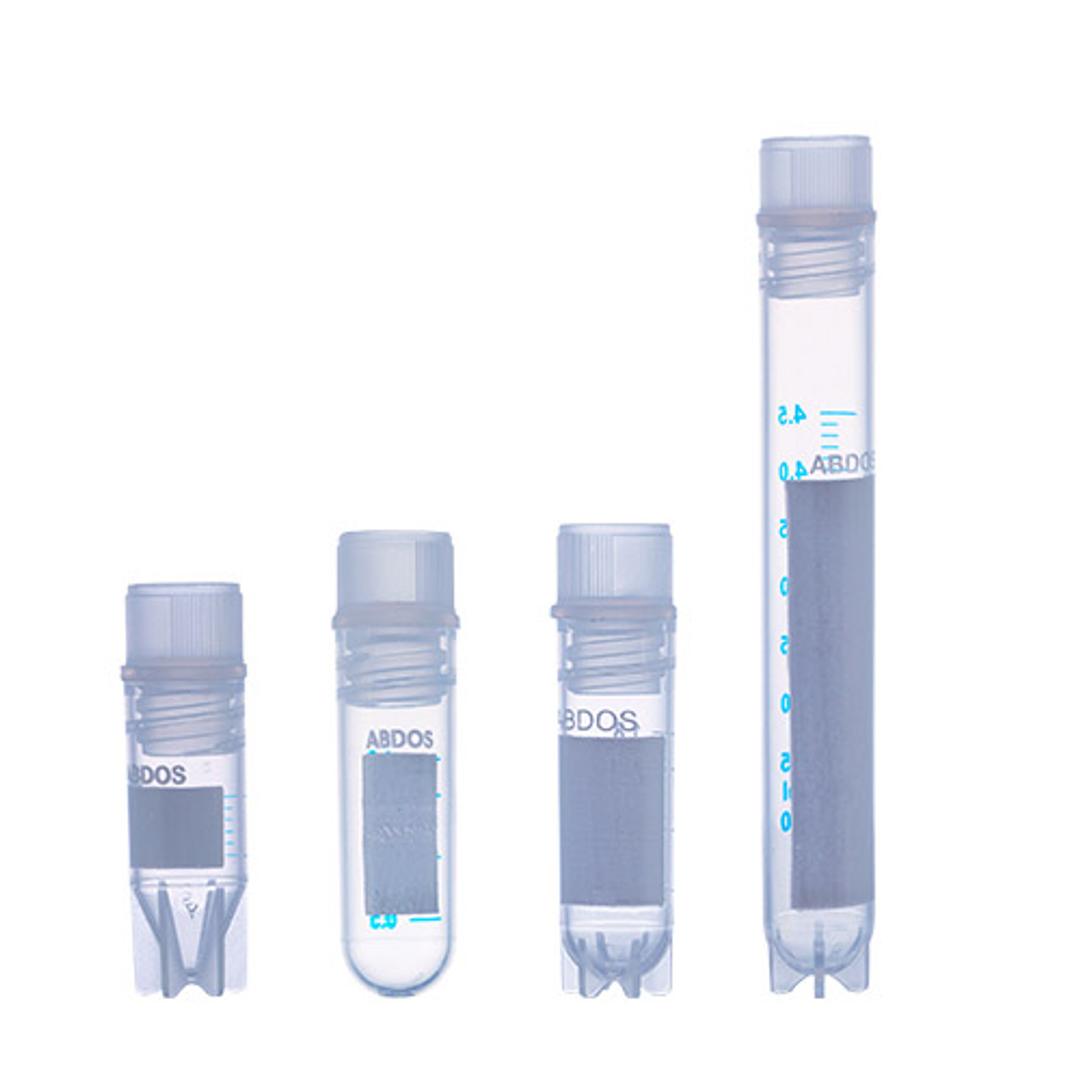
Cryo Vial Internal
Abdos Cryo Vial internal thread are manufactured using high purity virgin USP Class VI Medical grade polypropylene (PP) conforming to US FDA 21 CFR, free from natural rubber & heavy metal. Abdos Cryo Vial is designed for long-term cryogenic storage of biological samples, all type of cells and any enzymes or reagents. It’s leak-proof cap design with Silicon Seal and strong wall thickness makes it an ideal product for transp…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Good quality product
Store cell lines in liquid nitrogen as well as RNA, DNA, protein, cells and tissue samples at -80'C
ABDOS cryo vials are good quality product which is at par with the alternatives from well-known brands
Review Date: 14 Dec 2022 | Abdos
Abdos Cryo Vial internal thread are manufactured using high purity virgin USP Class VI Medical grade polypropylene
(PP) conforming to US FDA 21 CFR, free from natural rubber & heavy metal.
- Abdos Cryo Vial is designed for long-term cryogenic storage of biological samples, all type of cells and any enzymes or reagents.
- It’s leak-proof cap design with Silicon Seal and strong wall thickness makes it an ideal product for transporting samples and specimens.
- These cryo vials are designed for vapour phase LN2 freezing and also incorporate a silicone seal to enhance cap sealing.
- Manufactured to be used at a temperature between -196°C to 121°C.
- The large white frosted portion for easy writing.
- Graduation in silk-screen printing makes identification easy.
- Unique Star Foot design allows for single hand operation and grooves into Abdos P20616 single hand cryo holder rack.
- The silicon ring used in Abdos Cryo Vial for Sealing effects is made up of the material & ingredient under the code of federal regulation 21, as per 21 CFR 177.2600 (G) & meets USP – Class VI Regularities under plastic vial & Cap.
- Best suited for IVD use (an IVD Certified product).
- Sterile Tubes have been validated according to ISO 11137 for SAL 10-6