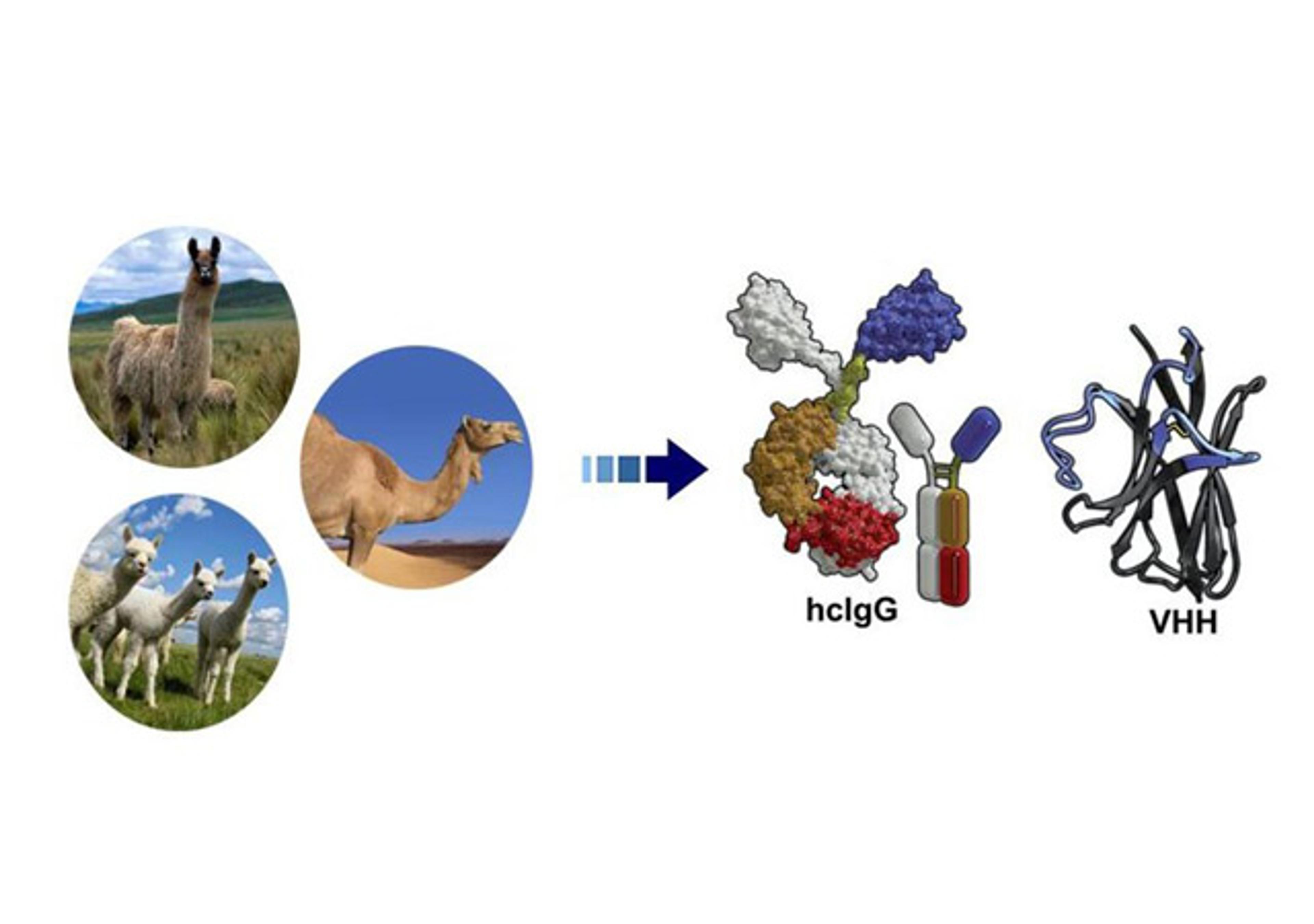
TIL Therapy Development Solutions
Configurable TIL therapy development spanning tumor processing, TIL isolation/rapid expansion, functional and phenotypic qualification, and release criteria—paired with manufacturing‑aligned process development and IND documentation to de‑risk scale‑up for solid‑tumor programs.
Creative Biolabs offers translational Tumor‑Infiltrating Lymphocyte (TIL) therapy development, delivering a configurable workflow from tumor tissue processing and TIL isolation/expansion to potency testing, phenotype profiling, and release criteria definition.
Manufacturing‑minded methods (e.g., rapid expansion protocols, cytokine/feeder strategies) and analytics (exhaustion markers, clonality, tumor reactivity) help de‑risk scalability while supporting IND documentation and technology transfer.
Key Features / Benefits
- Tissue dissociation → TIL isolation → expansion (REP) → functional qualification
- Phenotype, reactivity and clonality assessment; cytokine secretion and killing assays
- Manufacturing‑aligned process development and comparability packages
- Regulatory documentation support for IND‑enabling studies
Relevant Applications
- Autologous TIL programs for solid tumors requiring robust expansion and potency
- Method transfer, scale‑up, and release testing for clinical manufacturing
- Biomarker and mechanism studies on TIL phenotype and persistence