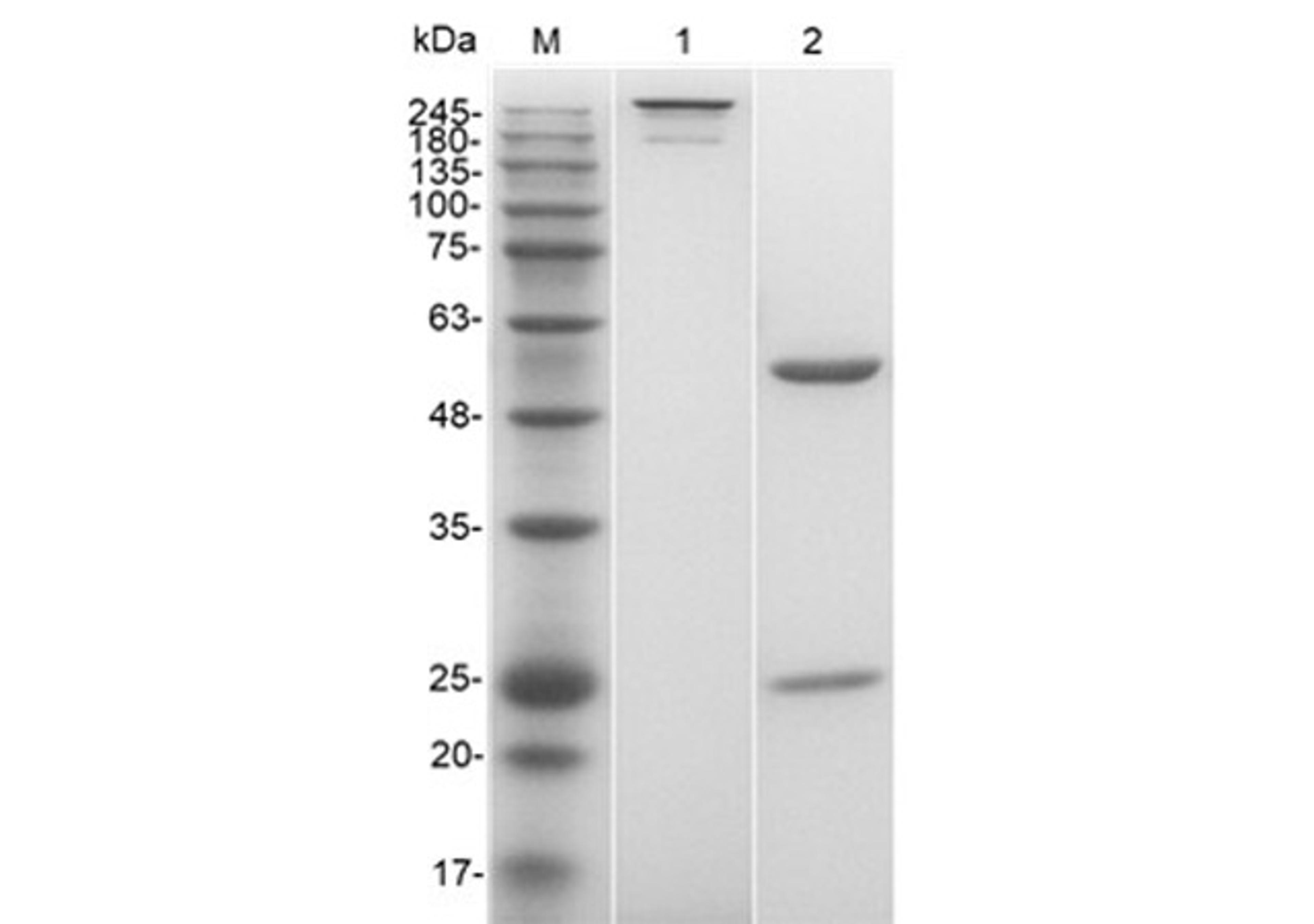
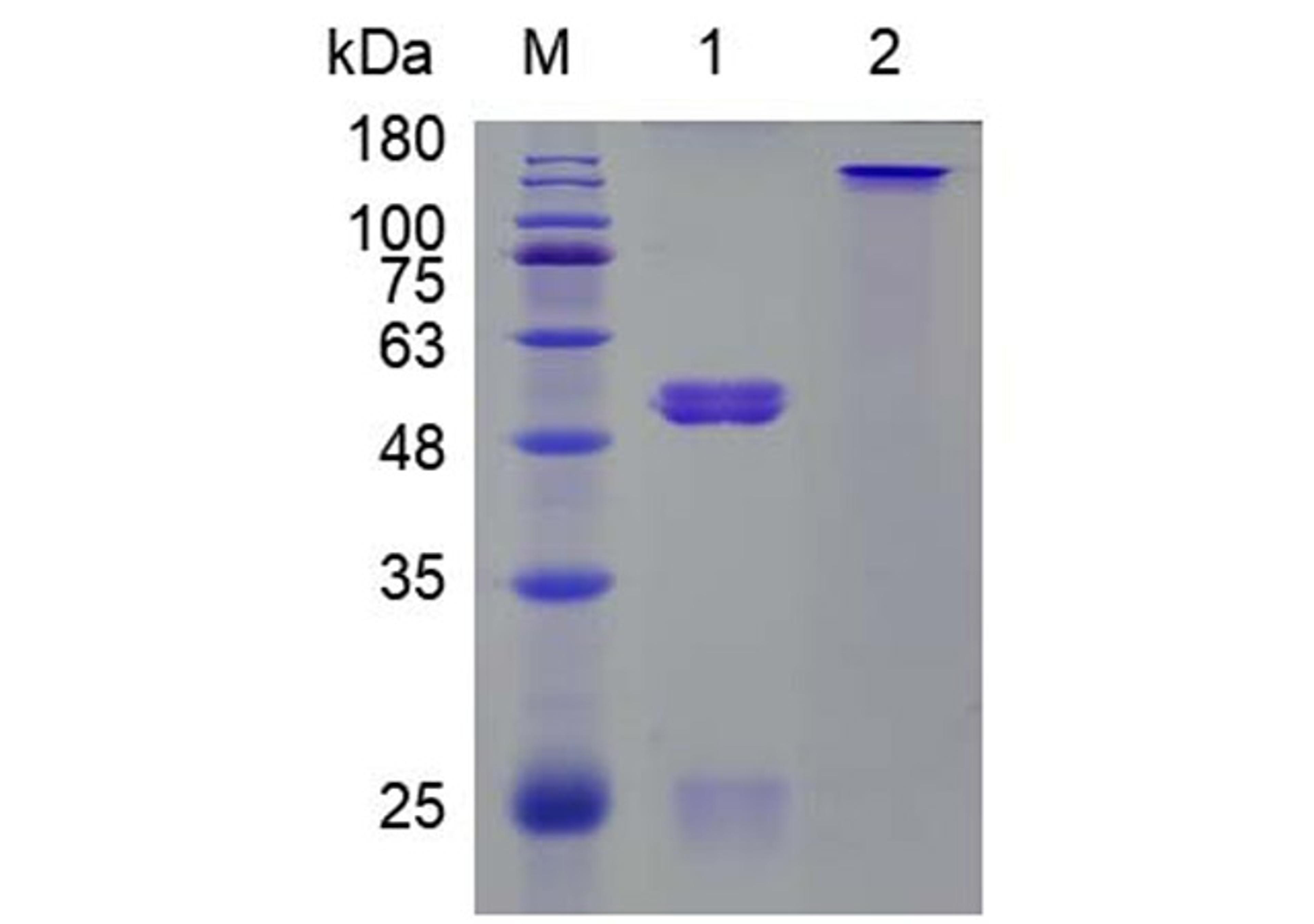
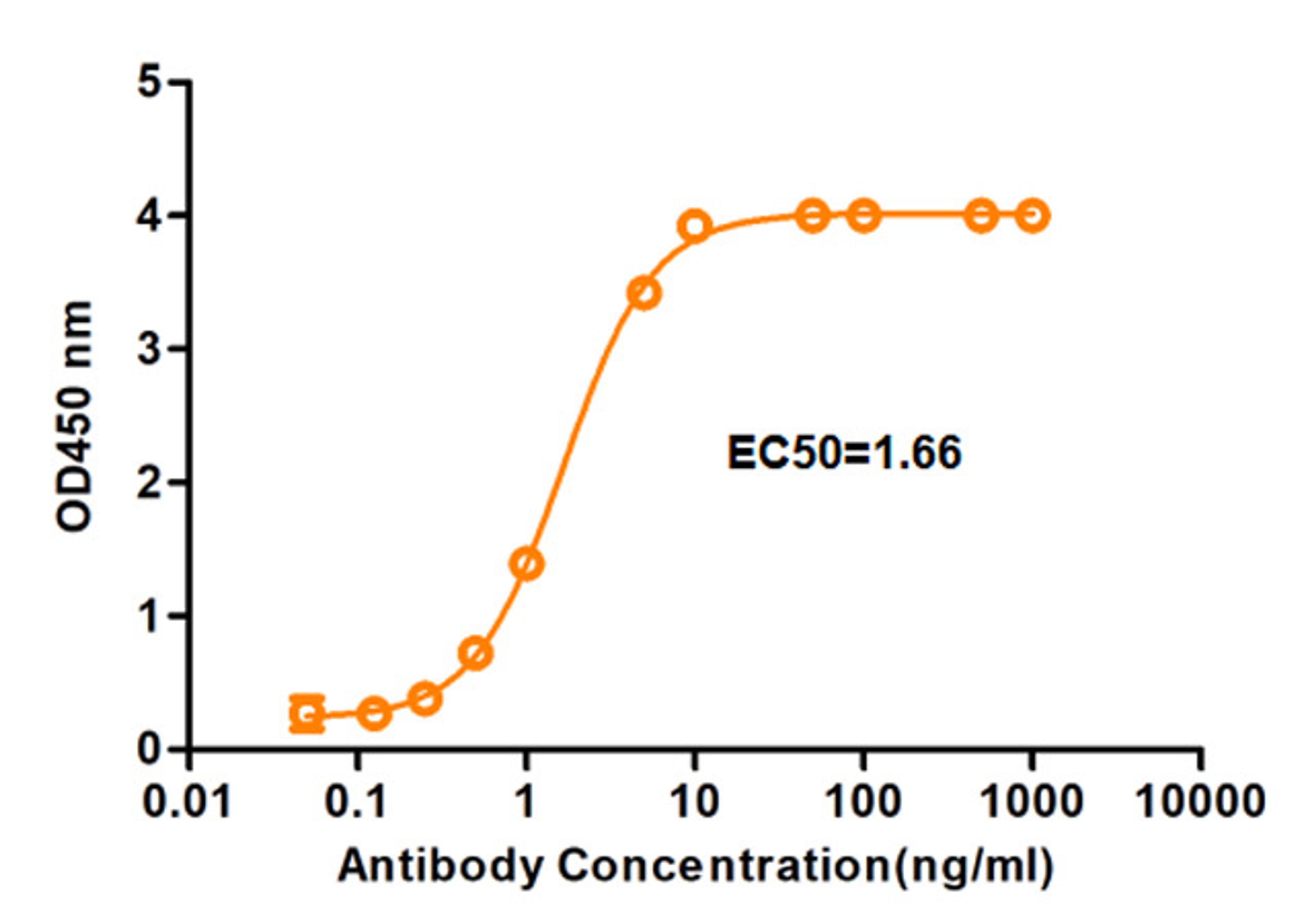
One-Stop CAR-T Therapy Development
Creative Biolabs delivers an end-to-end CAR-T development pathway—from target/scFv discovery, CAR design and vectoring, and cell engineering to in-vitro/in-vivo efficacy, IND authoring, and manufacturing support. Modular and GMP-aligned, the platform spans autologous and allogeneic routes (incl. TRAC/Cas9 and mRNA) to de-risk translation for hematologic and solid tumor programs.
Creative Biolabs delivers true end-to-end CAR-T development—spanning target/scFv discovery, CAR design and vector construction, cell engineering, in‑vitro/in‑vivo efficacy testing, IND dossier preparation, and manufacturing support—so you can streamline vendors, de‑risk tech transfer, and move faster to clinic.
Built on modular, rigorous quality practices, the program supports hematologic and solid‑tumor pipelines and accommodates advanced routes (allogeneic/TRAC‑Cas9/mRNA/γδ T) to address safety, consistency, and scalability.
Key Features / Benefits
- End‑to‑end workflow: discovery → CAR design/construction → cell engineering → in‑vitro/in‑vivo efficacy → IND → manufacturing support
- Multiple technology paths: allogeneic, TRAC/Cas9 knock‑in, mRNA/LNP, γδ T options
- Regulatory enablement: IND consulting and documentation preparation
- Modular services that integrate into existing pipelines
Relevant Applications
- Hematologic malignancy programs from discovery to IND
- Solid‑tumor CAR‑T with design optimization and robust efficacy packages
- Next‑gen modalities (mRNA/LNP, TRAC/Cas9, allogeneic) for manufacturability and access