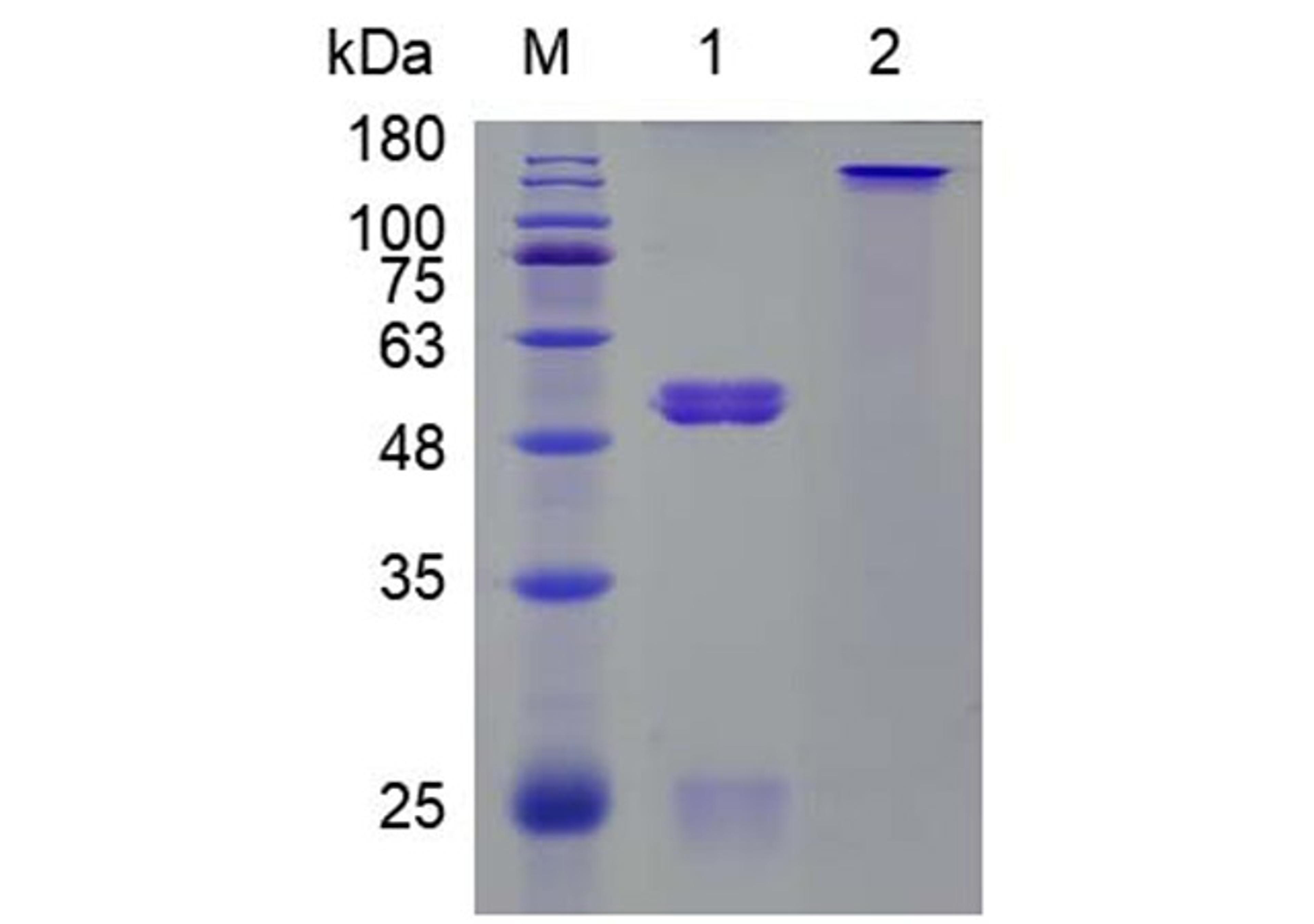
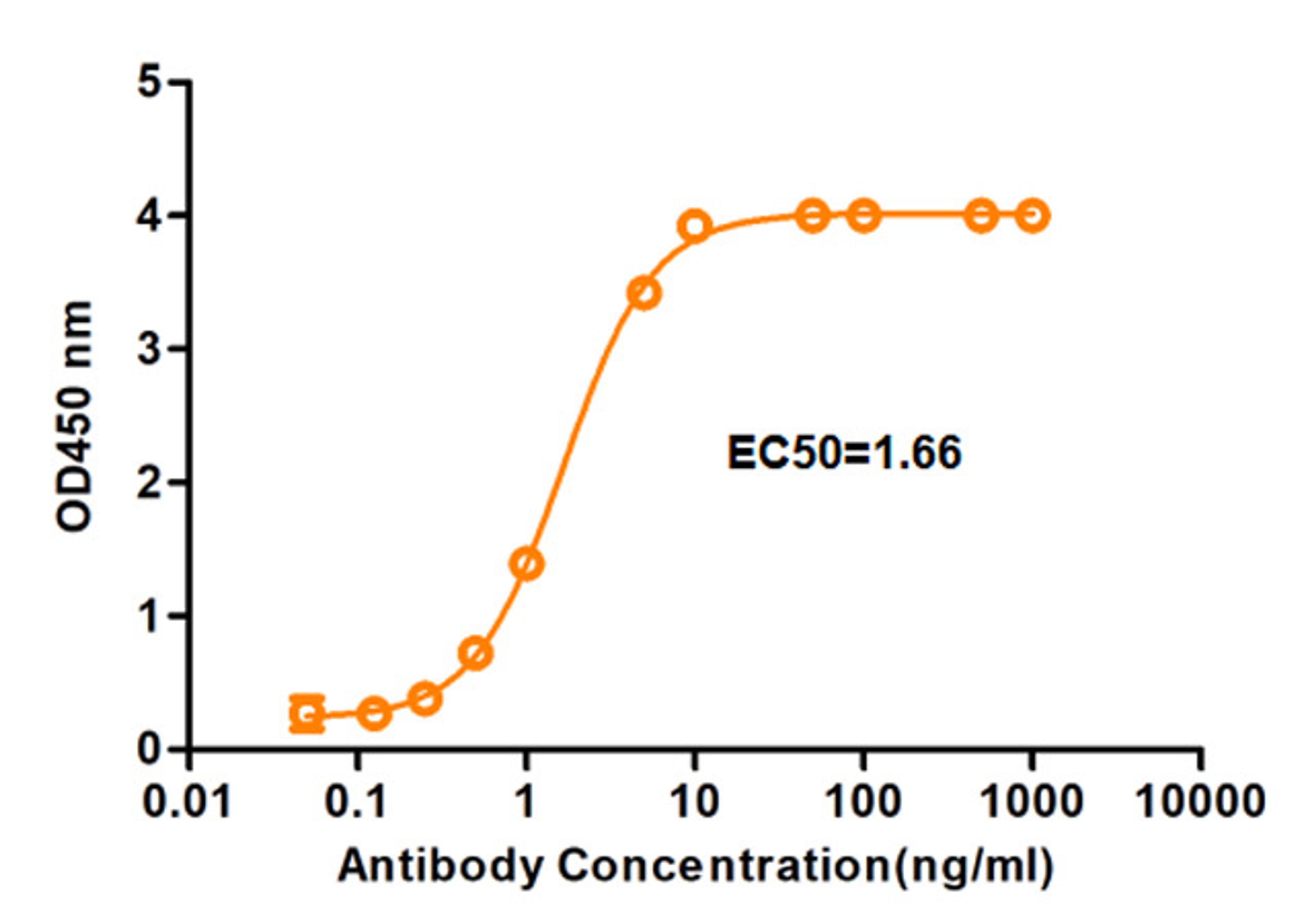
One-Stop Allogeneic Cell Therapy Development Solutions
End‑to‑end allogeneic cell therapy development—covering donor strategy, multiplex genome editing for safety/immune evasion, scalable manufacturing and comparability, and IND/CMC support—across CAR‑T/CAR‑NK and other donor‑derived modalities to enable off‑the‑shelf access.
Creative Biolabs supports one‑stop development of allogeneic, off‑the‑shelf cell therapies, enabling donor sourcing/qualification, genome editing for GVHD/immune evasion, and modular manufacturing designed for consistency, cost, and access.
The platform accommodates CAR‑T/CAR‑NK and other donor‑derived cell products, providing editing strategies (e.g., TCR/β2M/CIITA knock‑out, HLA engineering), scalability studies, and IND‑enabling documentation.
Key Features / Benefits
- Donor material strategy and qualification; iPSC or peripheral blood starting materials
- Multiplex genome editing for safety and immune evasion (TCR, B2M, CIITA, HLA)
- Process scale‑up and comparability for off‑the‑shelf manufacturing
- Regulatory/IND support and CMC documentation alignment
Relevant Applications
- Allogeneic CAR‑T/CAR‑NK programs targeting scalable, consistent supply
- Editing‑enabled safety/engraftment strategies for off‑the‑shelf products
- CMC and IND‑enabling packages for first‑in‑human