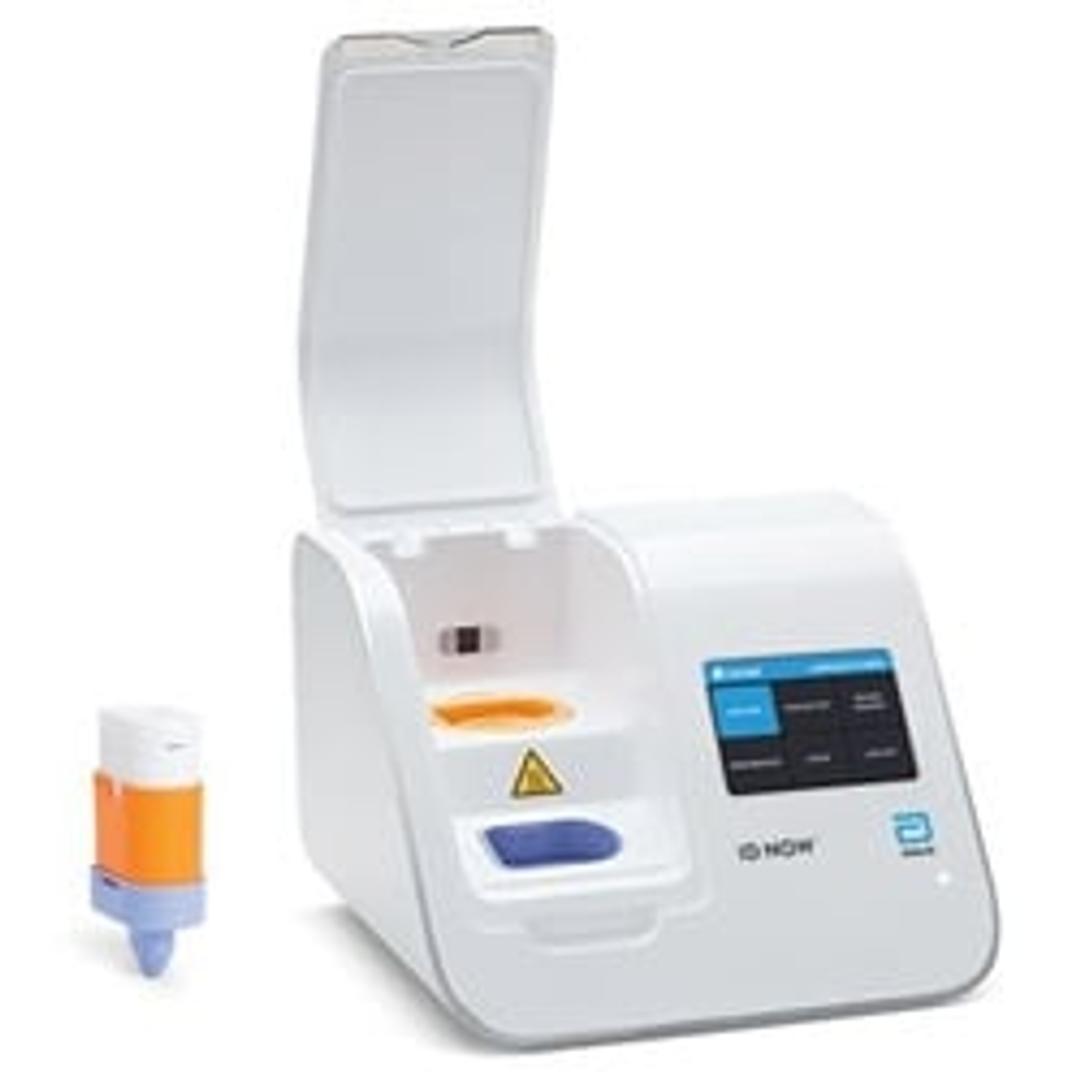
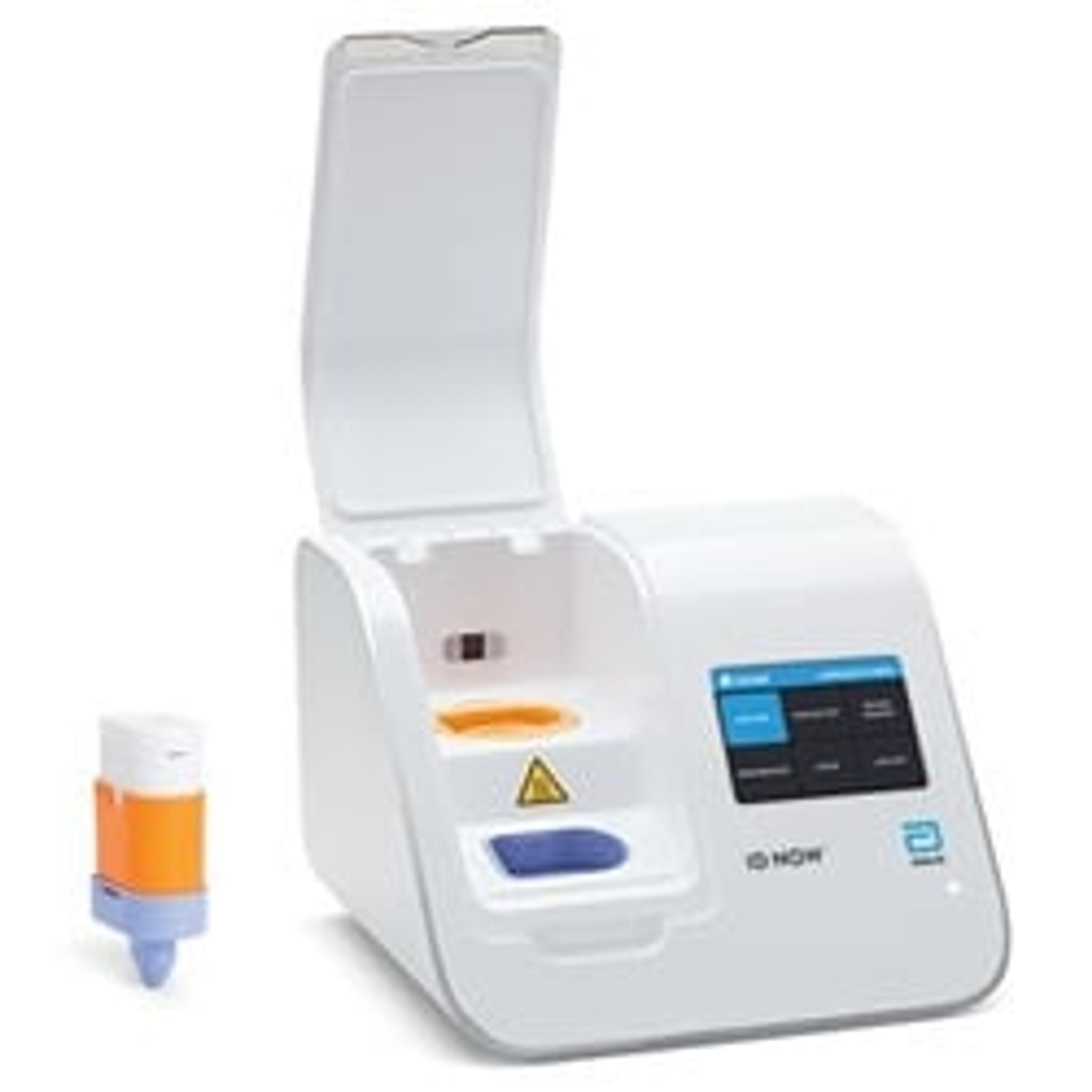
ID NOW™ Instrument (formerly Alere™ i)
A rapid, instrument-based, isothermal system for the qualitative detection of infectious diseases.
Love it and so easy to use
Testing samples for COVID-19
We received this placement analyzer and it has been a great addition to our in house testing for COVID-19. We may look at some of the additional testing available.
Review Date: 29 Mar 2022 | Abbott
Easy to use, easy to train new operators
Point of Care Testing
Great after purchase service, high ease of use.
Review Date: 20 Dec 2021 | Abbott
Really like the analyzer. we have several.
NP
Results are good, analyzer is very easy to use, not too long on test time.
Review Date: 16 Mar 2021 | Abbott
Supplies not available or on backorder.
Clinical
Easy to use and quick. Can't get supplies, they're always on backorder.
Review Date: 16 Oct 2020 | Abbott
Perfect outcomes and easy to use it.
Clinical
Excellent tool for research and clinical application.
Review Date: 22 Jul 2020 | Abbott
Ease of use is very good.
Metabolism tissue samples
Very effective in all manners, everyone should go for this product. It's reliable and cost effective. Ease of use is very good.
Review Date: 15 May 2020 | Abbott
Easy to use, but...
COVID-19 viral testing
This little analyzer is easy to use and gives quick results, especially compared to sending the samples out for testing, but we are a little skeptical about the LOD, especially since Abbott recently sent out a notice that "Negative results should be repeated with another method."
Review Date: 15 May 2020 | Abbott
Fast and easy to use for qualitative answers.
COVID-19 nasopharyngeal swab testing
This instrument is simple to use and includes step by step instructions on the screen to walk you through each test. Results seem reproducible and accurate so far, but I have only had the instrument for a month. Great for fast individual testing. Not recommended for volume test situations.
Review Date: 15 May 2020 | Abbott
Great instrument for COVID-19 tests.
Molecular tests
Excellent platform for COVID-19 molecular tests without special qualification.
Review Date: 10 May 2020 | Abbott
Our new favorite analyzer.
Molecular diagnostics
The training was extremely helpful, we were taught the old school way buy using scenarios that stick in your mind. We were using the ID Now as a confirmation on negatives that were run on a rapid kit. We found about 35% of negatives on rapid tests were positive on the ID NOW!
Review Date: 10 Feb 2020 | Abbott
ID NOW™ is a rapid, instrument-based, isothermal system for the qualitative detection of infectious diseases. Our unique ID NOW™ isothermal nucleic acid amplification technology provides molecular results in just minutes, allowing you to make effective clinical decisions sooner. ID NOW™ is significantly faster than other molecular methods and more accurate than conventional rapid testing.
Available Tests:
ID NOW™ Influenza A&B delivers molecular flu results in less than 15 minutes on our unique ID NOW™ platform. Rapid diagnostic tests with increased sensitivity are essential for the reliable detection of influenza A and B and enable immediate, effective treatment decisions.
ID NOW™ Strep A provides molecular results in 8 minutes or less on the unique ID NOW™ platform; allowing you to confidently test, and with a positive result, treat during the patient’s visit. The Strep A Molecular Test Now CLIA-Waived.
ID NOW™ RSV delivers molecular RSV results in 13 minutes or less on our unique ID NOW™ platform. Traditional laboratory methods and rapid antigen testing for RSV diagnosis have considerable shortcomings in terms of turnaround time or performance. ID NOW™ RSV detects 25% more true positives than Rapid Antigen Detection Tests (RADTs) and allows you to make real-time clinical decisions which impact patient care.
Features & Benefits:
- Easy to use with only minimal training requirements
- Large visual touchscreen displays results, eliminating transcription errors and the need for printing
- Small footprint saves you bench space and can be used in any healthcare setting
- Offers exceptional performance
- Eliminates interpretation and transcription errors
- Gives you the confidence to make clinical decisions sooner
- Facilitates effective patient management
- Enables prompt initiation of infection control measures
- Aids targeted antiviral therapy and Antimicrobial Stewardship
- Reliable near-patient testing reduces overall healthcare costs
TO GET MORE FROM YOUR POC TESTING, CONNECT IT.
Connect More, Share More, Worry Less
A web-based, open platform, AegisPOC™ lets you better manage and share data from your POC devices on one flexible, scalable middleware. AegisPOC provides you with the ability to integrate POC devices with LIS, HIS, EMR, quality management, user management and other systems. Not available in the USA.