The role of the complement system in infectious disease: Expert opinion on future diagnostic and therapeutic potentials
Explore the role of the complement system in infectious disease and discover the immunoassays that drive research, diagnostics, and therapeutic monitoring
11 Jan 2023

Prof. Reinhard Würzner, Deputy Head of the Institute of Hygiene & Medical Microbiology, Medical University of Innsbruck, Austria is an immunologist and medical microbiologist.
The complement system plays an important role in the humoral innate immune response against the pathogens that cause infectious diseases. When dysfunction occurs, individuals can be more prone to infection, or if unmodulated, overactivation of the complement system can damage healthy cells. Expanding our understanding of this complex, regulated system – comprised of over 30 proteins – holds the potential to improve both disease diagnosis and available therapies. In this article, we explore the role of the complement system in infectious disease.
To learn more about this subject, we spoke to expert, Prof. Reinhard Würzner, previous European Complement Network President and current Deputy Head of the Institute of Hygiene & Medical Microbiology, Medical University of Innsbruck, Austria. In this interview, Würzner discusses key biomarkers of complement activation, the development of complement-targeting drugs, the importance of good immunoassays for complement research, and a new research project investigating opportunistic infections in immunocompromised individuals.
Complement as a biomarker for diagnosis of infectious diseases
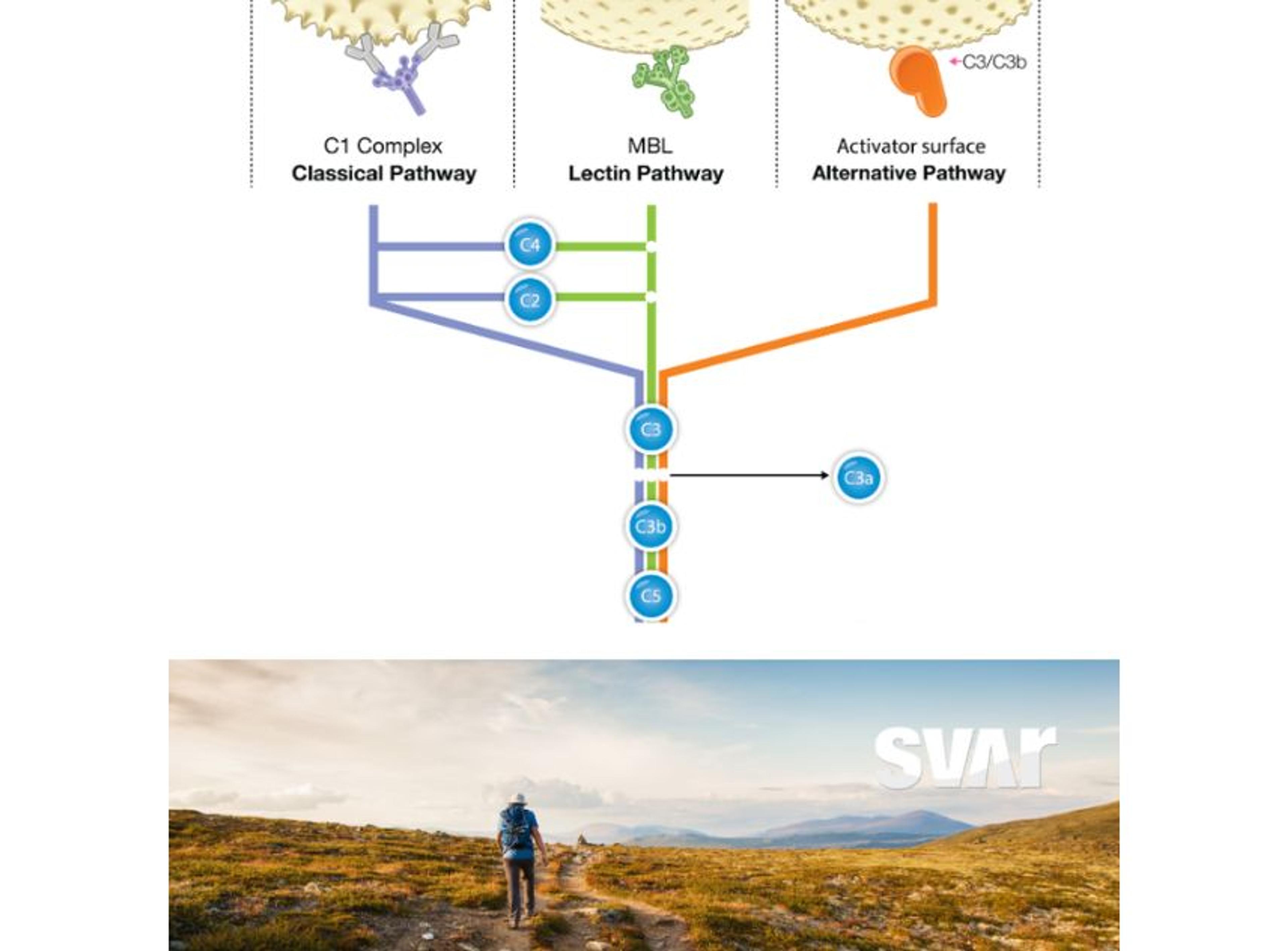
The complement system plays an important role in the immune response to many diseases, although the precise mechanisms of action have not always been clear. The system is very complex, with many regulators involved in its activation in the presence of an infectious disease. Consequently, the molecules involved in complement activation have the potential to serve as biomarkers that could be used to determine if deficiencies, overactivation, or other dysregulation of the complement system are present.
Molecules that increase greatly in the plasma upon activation of the complement system are of particular interest to researchers. One such biomarker is the terminal complement complex (TCC), which can be used to detect activation of the entire complement pathway. This makes it a more eligible candidate to use as a diagnostic biomarker compared to upstream components of the TCC, levels of which are decreased upon pathway activation. Würzner shares, “to detect activation of the entire complement pathway, it is better to measure the increase in TCC, rather than to see how much C3, C5, C6, and C7 has been consumed (and therefore decreased).”
Complement activation results in a huge increase in C4d, which makes it a very specific biomarker for the classical pathway.
Prof. Reinhard Würzner Institute of Hygiene & Medical Microbiology, Medical University of Innsbruck
C4d is another key biomarker of complement activation, which has been associated with autoimmune disease.1 “Complement activation results in a huge increase in C4d, which makes it a very specific biomarker for the classical pathway. A decrease of C4, the mother molecule is, by comparison, much more difficult to detect when this molecule is consumed by classical pathway activation, as levels will only be decreased by, let's say 10% or at most 20%” explains Würzner.
Role of complement-targeting drugs in treating infectious diseases in the clinic
Some complement system proteins have the potential to be used as infection biomarkers, but is there a place for the complement system in infectious disease therapies?
Characterizing dysfunction and dysregulation of the complement system in infectious disease is a key step in determining suitable treatment strategies. “When you have overactivation of the system, which you can see as an increased level of markers, or you detect defects in the complement system, such as identifying proteins which are missing, this information can be used to inform the start of treatment,” describes Würzner.
The distinct role that the complement system plays in a disease can determine the direction of therapeutic decisions. But is there a possibility for targeting the complement system itself as a treatment for infectious disease?
Würzner’s laboratory developed the first inhibitor of human complement, the forerunner of eculizumab – a therapeutic antibody used in the treatment of a range of diseases, including atypical hemolytic uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria (PNH). “In the late eighties we generated monoclonal antibodies, anti C5, C6, C7, and one of the anti-C5 monoclonal antibodies was shown to be highly inhibitory. This antibody was later bought and humanized to become a forerunner of eculizumab,” Würzner explains.
“It's very important to have these inhibitors and to continue to improve on them,” Würzner continues, noting that while it is very helpful to stop complement activation at this stage, complement activation is already taking place before the C5 stage. Würzner goes on to say “the future is in complement inhibition earlier on in the different cascades of the classical, alternative, or the lectin pathways. It's important to have more refined inhibitors. But it’s also important to have good tools to measure the effect of these inhibitors by quantitating TCC or split products in the circulation.
Studying the complement system in opportunistic infections of immunocompromised individuals
Another field of interest relating to further understanding the complement system is that of opportunistic infections. Opportunistic infections are infections which affect people who are immunocompromised. For example, healthy people may contract streptococci which can cause angina and a sore throat. However, an immunocompromised subject may suffer, due to the defective immune defense, from an opportunistic infection, such as a fungal infection of the mouth or other infections that would not usually cause an issue. Würzner notes, “these infections didn't appear 100 years ago because we didn't have immunosuppressive agents, and in those days people who were immunocompromised at birth would simply die. However modern therapy can make people immunocompromised, and this brings about infections which we didn't have decades ago.”
The discovery of individuals with deficiencies in the complement system has led to an enhanced understanding of the importance of the complement system in immune defense. Further, it has aided the consensus that the clinical diseases most frequently associated with complement defects include bacterial infections and autoimmune diseases.2 However, Würzner explains that the role of complement in these infections is still not well understood, which is why the competitive European project, Complement Regulation and Variations in Opportunistic Infections (CORVOS), was set up and subsequently funded by the European Union. CORVOS includes 15 different projects in the field led by 15 Ph.D. students from all over the world, working at 10 universities in Europe in the labs of complement experts.
The future of complement-targeting therapies in infectious disease
The development of the first complement-targeting therapy, eculizumab, in the mid-nineties led to an ability to inhibit complement. Würzner predicts that future research will focus on new complement-targeting drugs that will inhibit different stages of the complement system. For Würzner, his main target for focus is C7 – the only TCC component which is not predominantly synthesized in the liver and thus modulating locally. Developing improved inhibitors that target different pathways is the future for new complement-targeting therapies and this is an exciting area of potential for researchers to continue to explore.
The role of immunoassays in drug development, diagnosis, and response to treatment
It’s important to have good tools to measure the effect of these inhibitors.
Prof. Reinhard Würzner Institute of Hygiene & Medical Microbiology, Medical University of Innsbruck
It is clear that reliable assays are required for monitoring the complement system in infectious disease and Würzner notes that there are a number of companies in this space that are producing good assays. One company that is providing the research field with high-quality assays is Svar Life Science which is providing, among many other assays, the Complement C4d assay mentioned above and the Wieslab® Complement System Screen. Würzner was part of the consortium, led by Moh Daha, which developed this assay in the early years of this millennium, also as a European expert initiative. This functional assay for all pathways is used for the qualitative and semi-quantitative determination of the functionality of the classical, alternative, lectin, or terminal complement pathways, and the determination of complement deficiencies in human serum. SVAR has recently used income from this assay to introduce prestigious annual SVAR complement prizes.
References
Cohen D, Colvin R, Daha M, Drachenberg C, Haas M, Nickeleit V, Salmon J, Sis B, Zhao M, Bruijn J, and Bajema I. 2012. Pros and cons for C4d as a biomarker. Kidney International, 81(7), 628–39.
Botto M, Kirschfink M, Macor P, Pickering M, Würzner R, and Tedesco F. 2009. Complement in human diseases: Lessons from complement deficiencies, Molecular Immunology, 46(14), 2774–83.