Post-translational modification research offers new insights into health and disease
Advancements in antibody-based techniques present an efficient and powerful means by which to investigate post-translational modifications
23 Oct 2023
Antibody-based techniques have emerged as a powerful tool to use in conjunction with mass spectrometry (MS)-based proteomics to fuel research into the role of post-translational modifications (PTMs) in disease. Among the chaos of cellular processes, PTMs are defining elements that shape the complexity, functionality, and diversity of the proteome.
After protein synthesis, PTMs modify the protein’s chemical and structural aspects, profoundly impacting its activity, localization, and interactions. This modulation allows cells to responsively adjust to external stimuli and an evolving environment, overseeing vital survival and functional processes.
PTMs play a pivotal role in cellular functions, influencing disease etiology and progression. For instance, protein phosphorylation can prompt a cascade of cellular reactions. However, malfunctioning PTMs, like the hyperphosphorylation seen in Alzheimer's, can lead to severe health issues. Advanced technologies from the likes of RayBiotech means the potential for groundbreaking therapeutic interventions draws nearer to fruition.
This article highlights the importance of PTMs in disease research, and advancements in the detection methods used to study it. Embracing these evolving methodologies will undoubtedly further understanding and management of various diseases in the future.
Post-translational modifications can alter the 3D structure of proteins
Aberrant PTMs can impact disease
PTMs have surfaced as major players in the etiology and progression of several diseases.
Post-translational modifications (PTMs) refer to the covalent and enzymatic changes a protein experiences post-ribosomal synthesis. These changes involve adding or removing functional groups, cleaving peptide bonds, or forming new bonds between amino acid side chains. Such modifications can cause structural shifts in proteins, influencing their activity – this might activate or suppress enzymatic functions, change protein localization, modify protein interactions, or mark a protein for degradation.
PTMs have surfaced as major players in the etiology and progression of several diseases. While numerous PTMs are recognized, certain modifications, by virtue of their prevalence and implications, have drawn heightened attention from the scientific community. Here, we shine a spotlight on some of these key PTMs and their roles in disease research.
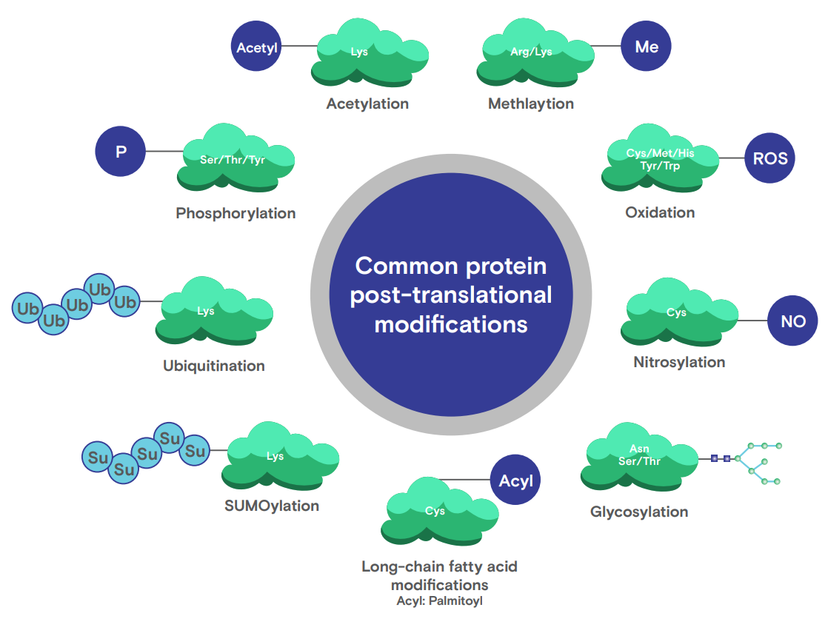
- Phosphorylation: The attachment of a phosphate group, often to serine, threonine, or tyrosine residues, is crucial for cell signaling. Dysregulated phosphorylation, like tau protein hyperphosphorylation in Alzheimer's, leads to issues like neuron degeneration. Additionally, altered phosphorylation in some proteins can trigger uncontrolled cell growth in many cancers.
- Acetylation: By adding an acetyl group, primarily on lysine residues, acetylation impacts protein interactions and gene expression. Imbalances can affect DNA accessibility and are linked to cancers and metabolic disorders due to aberrant gene expressions.
- Glycosylation: The addition of carbohydrate chains influences protein folding and cell adhesion. Abnormal glycosylation is observed in congenital disorders of glycosylation (CDGs) and some cancers, influencing cell adhesion and metastasis.
- Palmitoylation: The addition of fatty acids, mainly palmitic acid, to cysteine residues controls protein-membrane interactions and localization. Disruptions are associated with disorders like Huntington's due to protein mislocalization.
- Nitrosylation: Adding a nitric oxide group to cysteine residues affects protein function and signal pathways. Imbalances are tied to cardiovascular diseases through impacts on blood vessel dilation and blood flow.
- Oxidation: Incorporating an oxygen molecule can damage proteins. While naturally occurring, heightened oxidative modifications are associated with age-related and neurodegenerative disorders, such as Parkinson's, due to neuron damage.
Detection techniques that drive PTM research
Understanding the world of post-translational modifications (PTMs) requires a suite of sophisticated tools and techniques. The past few decades have seen significant advancements in this domain, with methodologies emerging that can detect and quantify these modifications with remarkable precision.
MS-based proteomics: Mass spectrometry (MS) has firmly established itself as the cornerstone in the study of PTMs. This technique allows for the identification and quantification of proteins and their post-translational modifications in biological samples. Once proteins are extracted from a sample, they are digested into peptides, which are subsequently separated using liquid chromatography. Upon entering the mass spectrometer, these peptides are ionized, and their mass-to-charge ratios are measured, enabling researchers to deduce the peptide's amino acid sequence and any modifications present. In the domain of disease research, MS has unveiled altered protein phosphorylation patterns in cancer cells, providing valuable insights into potential therapeutic targets.
Antibody-based techniques: Antibody-based methods offer complementary and accessible approaches to detect specific PTMs.
- Antibody arrays: Arrays enable simultaneous screening of multiple protein targets, each with its distinct PTM. RayBiotech's antibody arrays, renowned in the scientific community for their comprehensiveness, offer broad coverage of targets and PTMs. These arrays leverage the specificity of antibodies to capture and detect proteins with particular modifications, providing a snapshot of the PTM landscape in a given sample.
- PTM-specific antibodies: These are antibodies designed to recognize and bind only to proteins that possess a particular modification. For instance, an antibody developed against acetylated lysine can be employed to detect and quantify the levels of protein acetylation in a sample. This specificity allows for the precise targeting of specific PTMs, making these antibodies invaluable in research settings where researchers are keen to probe a particular modification in detail.
Antibody-based methods offer complementary and accessible approaches to detect specific PTMs.
Given the vast dynamic range and complexity of biological samples, directly analyzing them via MS can be challenging. Enrichment techniques are employed to isolate and concentrate specific PTMs, enhancing the detection sensitivity. For example, metal-affinity chromatography can be employed to enrich phosphopeptides, while acetyl-lysine affinity enrichment can capture acetylated peptides.
Often however, a single technique may not be enough to paint a comprehensive picture of the PTM landscape. Combining MS with antibody-based approaches can offer a holistic understanding. For instance, using PTM-specific antibodies to enrich for specific modifications before MS analysis can marry the specificity of antibodies with the depth of MS-based proteomics.
PTM-specific antibodies can help solve challenges in disease research
The journey of understanding post-translational modifications (PTMs) has not been without its challenges. While advancements have broadened our understanding, obstacles persist, emphasizing the need for innovative strategies.
Detecting low-abundance modifications remains a hurdle due to many PTMs' transient nature, often overshadowed by the dominance of non-modified proteins. Technical reproducibility, too, is a concern. The nuanced process of PTM analysis, encompassing everything from sample preparation to advanced detection methods, makes consistent results an aspiration. The introduction of PTM-specific antibodies has been pivotal, but their specificity is not foolproof. Cross-reactivity remains an issue, risking data misinterpretations. Finally, the intricate interplay between concurrent PTMs, such as how phosphorylation might influence acetylation, adds another layer of complexity. Decoding this intricate network and gauging its overall influence on protein function is a formidable undertaking.
What’s next for PTM research?
PTMs play a crucial role in protein regulation. They offer cells a rapid adaptability tool, allowing modification of existing proteins instead of time-consuming new protein synthesis. This mechanism adds great complexity and diversity to biology. For context, while the human genome encodes for around 20,000–25,000 genes, PTMs amplify the functionality of these proteins, allowing a single protein to have multiple roles based on its modifications.
There are a number of avenues research into PTM could take in the future:
Integration of techniques: The synergistic combination of various techniques, like merging MS-based proteomics with antibody-based methods, will likely become more prevalent. This approach offers the potential to enhance the depth, accuracy, and comprehensiveness of PTM studies.
Development of advanced algorithms: With the increasing complexity of PTM datasets generated, there is a growing need for sophisticated algorithms and software tools that can process, analyze, and interpret this data. Machine learning and artificial intelligence could play pivotal roles in future PTM data analysis.
Expanding the PTM lexicon: While many PTMs are well-documented, emerging research indicates that the world of PTMs is even more vast and varied than currently appreciated. Ongoing research will likely uncover novel PTMs and elucidate their biological significance.
Therapeutic implications: With a deeper understanding of PTMs, particularly their roles in diseases, there is potential to develop targeted therapies. PTMs could serve as therapeutic targets themselves or offer insights into modulating protein functions in disease contexts.
Single-cell PTM analysis: Given the heterogeneity within tissues and even within seemingly identical cells, there is a growing interest in single-cell PTM analysis. This approach could provide insights into cell-to-cell variations in PTM landscapes, particularly relevant in complex tissues or tumor microenvironments.
