Overcome the challenges of dried blood spot sampling with quantitative DBS technology
In this guest editorial, discover how Capitainer® DBS cards with qDBS technology enable easy self-sampling with precision and accuracy
15 Jan 2024
Dried blood spot (DBS) sampling is commonly used in newborn screening to detect various metabolic disorders and genetic conditions, allowing timely interventions to prevent or minimize potential health issues. However, conventional DBS cards lack volumetric control which results in poor precision and accuracy, limiting their clinical applications. To address these challenges, Capitainer, a Swedish medtech company, has developed DBS microsampling cards with volumetric control based on innovative quantitative DBS (qDBS) technology. This novel solution could expand the use of DBS technology and pave the way for a transition to self-sampling diagnostics. It has already proven successful in monitoring kidney transplant patients1, improving the comfort of aftercare for patients and alleviating pressure on healthcare services.
DBS sampling is often advantageous over liquid blood sampling due to its simplicity, convenience, and minimal invasiveness - it eliminates the need for venipuncture and the collection of large blood volumes. DBS samples also have greater stability and can be stored at room temperature, ensuring easier and more cost-effective storage and transportation, while also minimizing biohazard risks.
Despite the benefits of DBS sampling, it is currently limited to qualitative and semi-quantitative analyses such as newborn screening, where exact analyte concentrations are not crucial. DBS sampling is also utilized in other bioanalytical studies like toxicology, disease and drug monitoring, microbiome testing, and forensic analysis.
Capitainer DBS cards with qDBS technology address precision and accuracy limitations seen in traditional DBS cards. Dr. Donald Chace, a Principal Scientist at Capitainer and leading expert in newborn screening and clinical mass spectrometry, employs this cutting-edge technology to conduct disease and heavy metal screening in newborns, which has revolutionized his work. In this article, Dr. Chace shares how qDBS holds the potential to transform DBS sampling, paving the way for numerous global applications and enhancing the affordability and accessibility of self-sampling diagnostics.
The challenges of DBS sampling
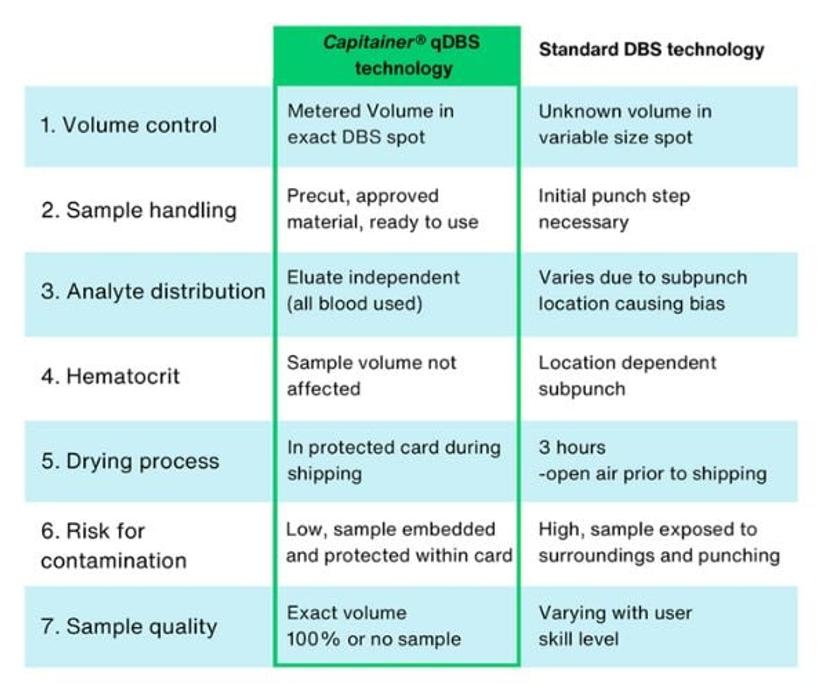
The absence of volumetric control in conventional DBS sampling results in imprecision and inaccuracies during analysis.
Dr. Donald Chace Principal Scientist at Capitainer
The DBS sampling process involves extracting a drop of blood from a finger or heel prick, blotting it onto filter paper, and leaving it to dry before analysis. Traditional DBS cards require a subpunch with a fixed diameter to obtain the blood spot for analysis. However, this process lacks precise volumetric control, resulting in substantial variability in blood sample volume. This variation is attributed to differences in the volume of blood drop applied to the paper and as well blood hematocrit (Hct) levels – a measure of the proportion of red blood cells in relation to the total blood volume. High Hct levels lead to increased blood viscosity, affecting how blood is distributed on filter paper. The volume of blood in the punched spot will therefore vary depending on the size of the original spot and its hematocrit.
Dr. Chace explains, “The absence of volumetric control in conventional DBS sampling results in imprecision and inaccuracies during analysis. In newborn screening, traditional DBS cards exhibit a high imprecision rate of 15–20%. This means they are not suitable for most clinical diagnostic tests which demand precise quantitative results to ensure accurate and reliable assessments.”
He continues, “Traditional DBS sampling methods also make it difficult to obtain a high-quality sample without professional assistance. It can be messy and inefficient, often requiring the collection of multiple spots to extract a single good sample, resulting in the wastage of blood. Additional challenges with traditional DBS sampling include a three-hour open drying process before shipping to the lab, which raises contamination risks as the sample is exposed. The subpunch step during the process may also introduce contaminants.”
qDBS technology enables volumetric dried blood sampling
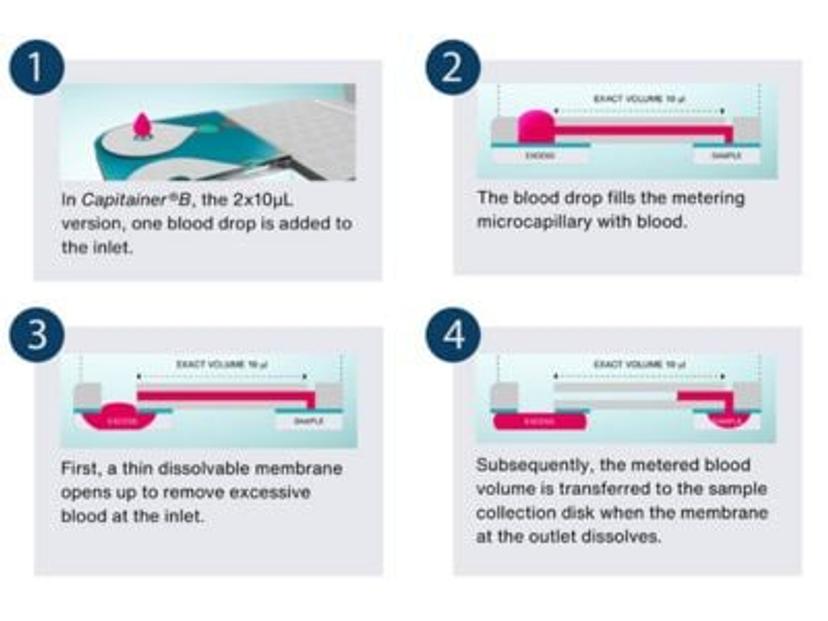
Capitainer has come up with an innovative solution to these challenges that involves using novel quantitative DBS (qDBS) technology, enabling easy, precise and accurate self-sampling with volumetric control. Capitainer DBS cards use a combination of paper, polymer microfluidics and thin water-soluble membranes to meter a fixed volume of blood from a single finger prick. After applying blood to the inlet, a metering channel in the device is automatically filled and the blood is transferred to a pre-cut DBS paper disc that is ready to use. This way, an exact volume (10 µL or 50 µL) DBS spot is automatically generated regardless of the size of the applied blood drop. If too little blood is added, the metering channel is not filled, and no blood is transferred to the sample disc. A clear no sample.
“Capitainer DBS cards eliminate the need for a subpunch and reduces the impact of hematocrit effects on blood volumes as the entire volume of blood is used for analysis. By addressing these challenges and enabling control over blood volume, this technology reduces imprecision of DBS sampling from 15–20% to 5%,” says Dr. Chace.
He explains, “I like to use a dart board analogy to explain precision, in which the DBS card is a dart, and the method used in sampling is the person throwing the dart. Capitainer, in this analogy, consistently hits the same area of the dartboard with high precision, outperforming competitors who exhibit greater variability in where their darts land.”
Capitainer DBS cards also offer a significant advantage in contamination prevention. After blood collection, the cards are securely placed in a paper-based drying pouch, allowing them to dry during transportation. This eliminates the need for a separate drying step, enhancing user convenience and substantially reducing the risk of contamination.
Dr. Chace emphasizes, “Every aspect of the Capitainer DBS cards is tailored for user ease and accessibility. This technology ensures the acquisition of high-quality quantitative samples, maintaining accuracy and precision comparable to liquid blood samples, but without the necessity for invasive venipuncture. It also utilizes a significantly smaller blood volume, enhancing overall efficiency.”
Newborn screening and beyond
Dr. Chace explains that the high precision of Capitainer qDBS technology helps minimize false positives in clinical analysis, which prevents unnecessary interventions and improves healthcare outcomes. In addition, the sample protection provided with qDBS reduces the risk of contamination from the surroundings. He elaborates, “In the mass spectrometry analysis of dried blood spots using conventional cards, measuring a specific mass-to-charge ratio (m/z) to a reference signal is essential for accurate and standardized measurements. However, as an observation, with Capitainer’s technology, the card's precision is so exceptional that the quality of the raw signal data itself without reference to a standard directly was quite precise. This almost certainly accelerates future assay development.”
The potential applications for Capitainer DBS cards stretch beyond newborn screening. This technology is also valuable for monitoring babies after discharge, especially those with conditions such as phenylketonuria (PKU)2, a disorder of amino acid metabolism, diabetes, or rare diseases. Accurate and precise monitoring is crucial in these cases to guarantee that patients receive the appropriate treatment.
Furthermore, a recent Norwegian study highlighted the applicability of Capitainer DBS cards in the routine monitoring of kidney transplant patients1. The research demonstrated equivalent sampling success and analytical quality for samples collected by healthcare staff in a hospital setting and by patients themselves at home. Integrating self-sampling into aftercare procedures can improve the convenience and comfort for kidney transplant patients while alleviating the strain on healthcare services.
Dr. Chace says, “I envision a future where Capitainer cards and qDBS technology becomes commonplace, with global applications ranging from routine screening, clinical chemistry, disease monitoring, nutrition, forensic science, cancer diagnostics, and even environmental health, including pollution monitoring.
I envision a future where Capitainer cards and qDBS technology becomes commonplace, with global applications ranging from routine screening, clinical chemistry, disease monitoring, nutrition, forensic science, and even environmental health.
Dr. Donald Chace Principal Scientist at Capitainer
Revolutionizing diagnostics and patient lives
Effective diagnostic self-sampling devices hold the potential to revolutionize healthcare and enhance patient lives. In the post-COVID era, where individuals are accustomed to performing various tasks from home, the convenience of at-home sample collection is a substantial advantage. Dr. Chace says, “This self-sampling capability aligns with Capitainer's mission to make diagnostics easily accessible, especially in remote or under-resourced areas like where storing or transporting liquid blood may not be feasible. For example, the ease and simplicity of these DBS cards could be particularly beneficial in military settings or even space exploration!”
He continues, “What’s more, improved accuracy, precision, and reproducibility in DBS analysis will empower multiple laboratories to produce consistent and reliable data. This, in turn, will assist physicians in making more informed, data-driven decisions, ultimately leading to better treatment outcomes for patients.”
To fully realize the potential of Capitainer qDBS technology, the next challenge is achieving broader acceptance. Dr. Chace emphasizes the importance of conducting validation assays to demonstrate that Capitainer DBS cards match the quality of blood samples obtained through traditional clinic-based methods. “These assays play a crucial role in bridging the gap between technology development and practical application. Other challenges include the necessary advancement of other technologies, such as immunoassay platforms and mass spectrometry tools, to reliably and accurately analyze smaller blood samples, as these tools are originally designed for larger blood volumes.”
Despite these upcoming challenges, Dr. Chace is optimistic that as technology advances, Capitainer cards with qDBS technology will gain broader acceptance. He concludes by stating that they are actively working on a pipeline of other products that could expand the technology's application, and that ongoing developments are in progress to enhance current cards and introduce additional benefits.
This guest article was written by Dr. Donald Chace, Principal Scientist and Senior Application and Product Specialist, Capitainer.
References
Vethe, N.T., Åsberg, A., Andersen, A.M., Heier Skauby, R., Bergan, S. and Midtvedt, K., 2023. “Clinical performance of volumetric finger‐prick sampling for the monitoring of tacrolimus, creatinine and haemoglobin in kidney transplant recipients.” British Journal of Clinical Pharmacology, 89(12), pp.3690–3701.
Carling, R.S., Emmett, E.C. and Moat, S.J. "Evaluation of volumetric blood collection devices for the measurement of phenylalanine and tyrosine to monitor patients with phenylketonuria." Clinica Chimica Acta 535 (2022): 157–166.
