Non-viral vectors transform gene delivery for cell therapies
Discover a novel non-viral gene delivery system that could transform cell and gene therapy workflows
4 Mar 2024
Cell and gene therapies are set to become a new standard of healthcare but are complex and difficult to manufacture at scale. This has prompted technology providers to develop new approaches – including the exploration of non-viral vectors for gene delivery – that tackle long-standing challenges and help to move therapies forward from the bench to the bedside.
How to overcome gene delivery challenges with non-viral vectors
The greatest challenge for cell therapy development remains the safe, effective, and reliable delivery of therapeutic genes into target cells. The industry standard approach to date has been – and remains – the use of viral vectors, with a majority of the gene therapies so far approved by the FDA using these vehicles. However, in recent years the limitations of viral vectors have driven technology providers to develop alternative non-viral approaches. These bring fresh challenges, but the successful deployment of emergency-use COVID-19 vaccines harnessing mRNA-based lipid nanoparticles has ushered in excitement and a new hope of developing effective and safe non-viral vector-based gene therapy products in the future.
In this editorial, we review the advantages of non-viral vectors over viral gene delivery systems. In particular, we explore a novel and exciting non-viral system using transposons.
Limitations of traditional viral vector gene delivery systems
Viral vector systems, such as lentiviruses, retroviruses, and adeno-associated viruses, continue to dominate clinical trials of new cell and gene therapies. However, they have many limitations and are far from easy to work with.
At the molecular level, viral vectors do not insert randomly into the target genome but integrate preferentially into more active coding regions. This can lead to insertional mutagenesis, disrupting the regulation of gene transcription.
At the more practical level, viral vector transfer capacity is limited to small cargo sizes. Additionally, long manufacturing timelines and batch-to-batch inconsistencies often lead to scale-up issues. The search for better alternatives has been underway for some time.
Non-viral vector gene delivery systems
An wide array of non-viral delivery systems is now available, offering lower cytotoxicity, immunogenicity, and mutagenicity compared with viral vectors. Additionally, manufacturing these non-viral delivery systems is typically much easier, cheaper, faster, and more consistent than producing and packaging viral vectors.
While they continue to show great promise, early non-viral vectors have faced challenges concerning gene transfer efficiency, specificity, expression duration, and safety. Some of these problems have been overcome by new technologies, such as CRISPR mediated knock-in systems, but their adoption has been limited by concerns over double-strand breaks, inflexible licensing terms and a complicated IP landscape. This has paved the way for further innovation in non-viral gene delivery systems, leading to the latest transposon-based mechanisms.
Consideration
Viral vectors
Non-viral vectors
Structure and delivery mechanism
Modified versions of viruses that are introduced by transduction of host cells, for example lentiviruses (LVVs), adenoviruses (AdVs), or adeno-associated viruses (AAVs)
Typically synthetic or natural materials, introduced by either physical vectors (e.g. electroporation) or chemical vectors (e.g. lipid nanoparticles)
Safety and immunogenicity
May trigger an immune response, resulting in potential safety concerns
Considered safer and well tolerated, with lower immunogenicity
Transfection efficiency
Offer high gene transfer efficiency
Generally lower efficiency and requires some optimization
Therapeutic application
Retroviruses and lentivirus can integrate their genetic material into the host genome, providing stable and sustained gene expression.
Offer diverse and versatile application for a wide range of cell types and genetic cargo. Depending on the cargo, can integrate into the host genome or provide transient expression. The latter may require repeat administration.
Manufacturing complexity
Require complex manufacturing processes often resulting in batch-to-batch inconsistency
A more simplified manufacturing process
Key differences between viral and non-viral vectors, including safety, efficiency, and delivery mechanism
A transposon-based gene delivery system
The TcBuster™ system from Bio-Techne is delivered via electroporation - pulses of electricity which create temporary pores in a cell membrane – to introduce both the transposase encoding mRNA, as well as the transposon (plasmid) and integrate the cargo gene sequence into the target cell. This solution decreases cargo size constraints and reduces mutagenic potential as the transposon system has a more random insertional profile in the target genome. Thus, the likelihood of disrupting normal gene transcription in the target patient cell is reduced.
TcBuster™ Non-Viral Transposon-Based Gene Delivery System
TcBuster is a non-viral transposon system that enables stable gene transfer into mammalian cells, supporting rapid and scalable generation of transgenic cells for cell therapy and bioprocessing applications. Find out more about TcBuster here.
Overall, this system presents a virus-free way of over-expressing nearly any protein of interest in a mammalian cell line, by offering large cargo capacity and robust, stable integration over many other approaches. The system can efficiently deliver both smaller single CAR constructs and larger-sized multicistronic constructs into target cells, showing an integration efficiency of between 50 and 70% for a single construct into a primary T cell. For multicistronic vectors encoding three or four transgenes, an integration efficiency of between 25 and 40% can be achieved.
In comparative studies with lentiviruses, the TcBuster system outperformed lentiviruses when inserting larger constructs into primary human T cells, and showed a safer integration profile. Furthermore, cytotoxicity and immunogenicity profiling revealed much lower cellular responses for those cells modified with the TcBuster transposase.
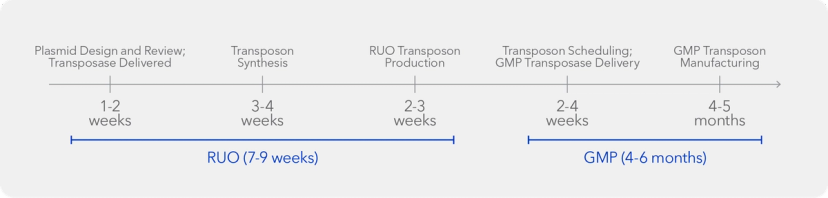
Furthermore, manufacturing nucleic acids is much simpler than viral vectors, with lower costs, shorter production timelines, greater consistency, and simpler scaling from RUP to GMP quality.
Unlike other non-viral gene delivery systems, such as CRISPR and other transposon systems, the TcBuster system is a commercially licensable technology with a clear IP landscape. It therefore provides developers with the freedom to operate and flexible licensing terms.
For these many reasons, some early adopters have already started clinical trials using TcBuster for non-viral gene delivery, paving the way for greater success to come.
Overall, the TcBuster provides non-viral gene delivery of larger cargos, with less risk, and in shorter manufacturing timelines than viral-based options and can be used with a variety of cell types, such as T cells, NK cells, induced pluripotent stem cells (iPSCs) for cell therapy development.

