Modelling the Complexity of the Gut Epithelium to Find Therapeutics for Gastroenteritis
Discover how Dr. Nicholas Zachos, Johns Hopkins Medicine, uses patient-derived stem cells to create complex co-cultures with the aim of developing new therapeutics to prevent diarrheal disease
25 Jul 2017

Nicholas Zachos, Ph.D., Assistant Professor of Medicine at Johns Hopkins Medicine, USA, is using novel intestinal stem cell culture techniques to re-create the human gut epithelium in vitro. Dr. Zachos is using this gut epithelial model to learn more about the causes of diarrheal disease, one of the biggest killers of children in the developing world, and to investigate potential therapeutics.
Starting out his career as a developmental biologist, Dr. Zachos became interested in studying the development of the polarized cells in the gut and the interaction between the epithelium and the gut microbiome.
“I want to investigate what influences the dynamic relationship between the membrane that faces the luminal environment and the microbial community that exists in our gut,” Dr. Zachos tells SelectScience.
Dr. Zachos is studying a range of infectious diseases which cause gastroenteritis, for example rotavirus and cholera.
“In developing countries, enteric pathogens such as rotavirus and cholera are persistent infections, that may be contracted over and over again. This can ‘reprogram’ the gut and alters the microbiome, which contributes to the development of environmental enteropathy,” explains Dr. Zachos. “In children, these infections can have long-term consequences for nutrient uptake and quality of life, leading to impaired physical and intellectual development. Their prognosis for a healthy life is poor.”
Dr. Zachos set out to investigate this by creating a model of the gut epithelium from cancer-derived or animal-derived cell lines, but it became apparent to him that there was a flaw in this approach.
The value of patient-derived cells
“The FDA (Food and Drug Administration) has recently reported that ~90% of drug development fails at the stage of human clinical trials. One of the reasons such a large proportion fail is because basic science studies are carried out in non-human or cancer-derived cells. These studies don’t always lead to the right targets or effective lead compound,” explains Dr. Zachos.
In response to this, Dr. Zachos’ laboratory is working with real patient samples. Using ex vivo stem cells from patients allows his team to create cultures which mimic those that occur in the patient, preserving patient-specific phenotypes. “This has been a really exciting advance and has allowed our field to move forward with understanding biological diversity and characterizing epithelial phenotypes related to various intestinal disorders.”
Dr. Zachos and his team use Corning® Matrigel® matrix to re-create the extracellular matrix proteins, which enables the patient-derived stem cells to grow, proliferate and form a 3D structure within the Matrigel matrix.
“Matrigel is a critical factor in allowing these cultures to propagate and mature,” says Dr. Zachos. “We found that the human intestinal stem cell cultures are difficult to grow. With Matrigel, we saw consistent growth rates over time and our human cultures seem to do much better on it than any of the other matrices we’ve tested, so we’ve stuck with it all these years and it’s given us success.”
The Corning® Matrigel® matrix is really a critical factor in allowing these cultures to propagate.
Modeling the gut epithelium
Dr. Zachos’ laboratory has adopted a novel methodology creating a model of the gut epithelium. The team begin by culturing enteroids – multi-lobulated, spherical cell structures – from patient-derived intestinal stem cells. In order to easily access the luminal-facing membrane, they then convert the enteroids into 2D monolayers. This monolayer allows them to mimic in vitro the interaction between the gut epithelium and gut microbiome.
Dr. Zachos and his colleagues convert 3D enteroids to a 2D monolayer by coating Transwell® permeable supports from Corning with collagen.
“We use the Transwell permeable supports as Corning Life Sciences offers them with a polyester membrane. The polyester membrane enables us to visualize the development of the monolayer using phase-contrast microscopy. We are able to see the cells going from a state which is more proliferative to a more mature state, which mimics that of the villous epithelium.”
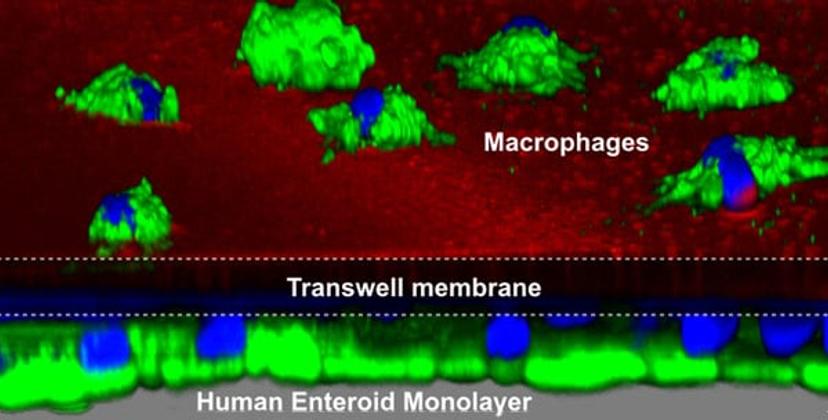
Dr. Zachos then investigated co-culturing immune cells alongside the epithelial cells to begin to study the way the innate immune system interacts with the gut epithelium and pathogens. In the 2017 paper published in Scientific Reports, the team showed how they could co-culture primary macrophages on one side of the Transwell permeable support insert and the epithelium and microbes on the other. This study revealed a synergistic relationship between the macrophages and the epithelium.
“We could see the macrophages responding to the bacteria and extending projections through the pores of the Transwell inserts and up in between the epithelial cells to reach across and phagocytose the bacteria in the lumen. These studies required Matrigel matrix to propagate the enteroids and then the Transwell permeable supports to allow us to produce this co-culture system,” explains Dr. Zachos.
Development of therapeutics
We’re using our enteroid model as a validation platform for drug discovery.
Dr. Zachos and his team have a multi-centered drug development grant funded by the NIH (National Institutes of Health) that enables them to collaborate with medicinal chemists and pharmacologists with the goal of developing new therapies to treat gastroenteritis.
“There are no good therapies to treat moderate-to-severe diarrhea, especially in developing countries, where oral rehydration solution is the only option. We’re using our enteroid model to link our findings in patient specimens and to remodel the disease phenotypes; using it as a validation platform for drug discovery,” says Dr. Zachos.
“In this research, our collaborative team uses human enteroids exposed to enteric pathogens (e.g. cholera and E. coli) to establish the altered epithelial response, and then tests different small molecule drugs to correct it,” says Dr. Zachos. In 2017, this team of collaborators published a paper in the FASEB Journalin which they used the enteroid model to validate the efficacy and potency of a new small-molecule CFTR (cystic fibrosis transmembrane conductance regulator) inhibitor. The findings showed that it has the potential to be used as a new anti-diarrheal drug.
Collaboration and future research
Dr. Zachos also runs the ‘Enteroid Core’ at Johns Hopkins Medicine. The purpose of the Core is to train other academics and industry professionals to culture, grow and use the enteroid models.
Dr. Zachos’ own group hopes to further develop this model. “Our future goal is to continue to push the limit of these models and to see how many diseases we can recapitulate in order to really understand what is happening at time of infection,” Dr. Zachos tells SelectScience.
“We want to understand how infections induce changes in the epithelium and how this affects the innate immune response. If we can gain a better idea of what happens at the early stages of infection, we can come up with targeted therapies to reduce the severity and increase the speed at which the patient can recover from these types of infections.”
Find out more about the Corning Matrigel and Transwells by visiting the Corning Life Sciences profile.
Do you use Corning Life Sciences products in your research? Write a review for your chance to win an Amazon voucher worth $400 or an iPad.