Tissue Regeneration – Unraveling the Most Remarkable Physiological Phenomenon, Cell by Cell
Regenerative medicine research scientist reveals how new single-cell sequencing technologies are helping to reveal the mechanisms of tissue regeneration in exquisite detail
16 Jul 2019

Dr. Brian Pepe-Mooney is a post-doctoral research fellow based at the Brigham and Women’s Hospital in Boston, in the laboratory of Dr. Wolfram Goessling, where his primary research interests lie in unearthing the molecular basis of tissue regeneration.
With a profound ability to self-restore structural and functional damage post-injury, Pepe-Mooney’s research focuses on the liver - and how its remarkable plasticity provides an exciting platform for the development of novel cancer therapeutics. Driven by personal experience and inspired by the unknown, Pepe-Mooney speaks with SelectScience to discuss his work in the field of regenerative medicine, and to explain exactly why the liver is a true ‘physiological phenomenon’. He reveals how some of the exciting findings from his doctoral work in Dr. Fernando Camargo’s laboratory, Boston Children’s Hospital, have enabled him to begin to understand its remarkable regenerative capacity.
SS: How did you become involved in this area of research? What inspires you?
P-M: I initially became interested in understanding the development and progression of cancer due to experiences with disease in my own family. What continues to inspire me, however, are the scientific advances made every day towards future regenerative-based therapies that may have a profound impact on patient lives. Regenerative medicine is such an important area of research right now due to the continued global need to combat human disease. By understanding the basic biological mechanisms that regulate regeneration and stem cell activity, we can seek to utilize this knowledge to better develop therapies for cancer, tissue replacement, or degenerative disease.
I am intrigued by the liver in particular because of its hugely diverse physiological functions and extraordinary ability to respond to injury - the liver has more than 500 vital roles. It serves as the main filtration system for the body, detoxifying components of blood from the intestine, and plays a central role in metabolism, synthesizing and secreting important hormones, and producing crucial molecules that aid in the processing of fats.
Studies have shown that up to two-thirds of a liver can be surgically removed, with what’s remaining quickly compensating for the loss of the tissue by undergoing rapid regrowth. This astounding regrowth occurs from the most abundant cell type within the liver - the hepatocyte. In scenarios of extreme injury, where hepatocytes are unable to compensate, another dramatic regenerative event takes place where cells from the biliary duct, biliary epithelial cells, transform into hepatocytes to functionally compensate for the liver damage. It’s truly amazing.
SS: What have been your major findings so far, and what projects are you currently working on?
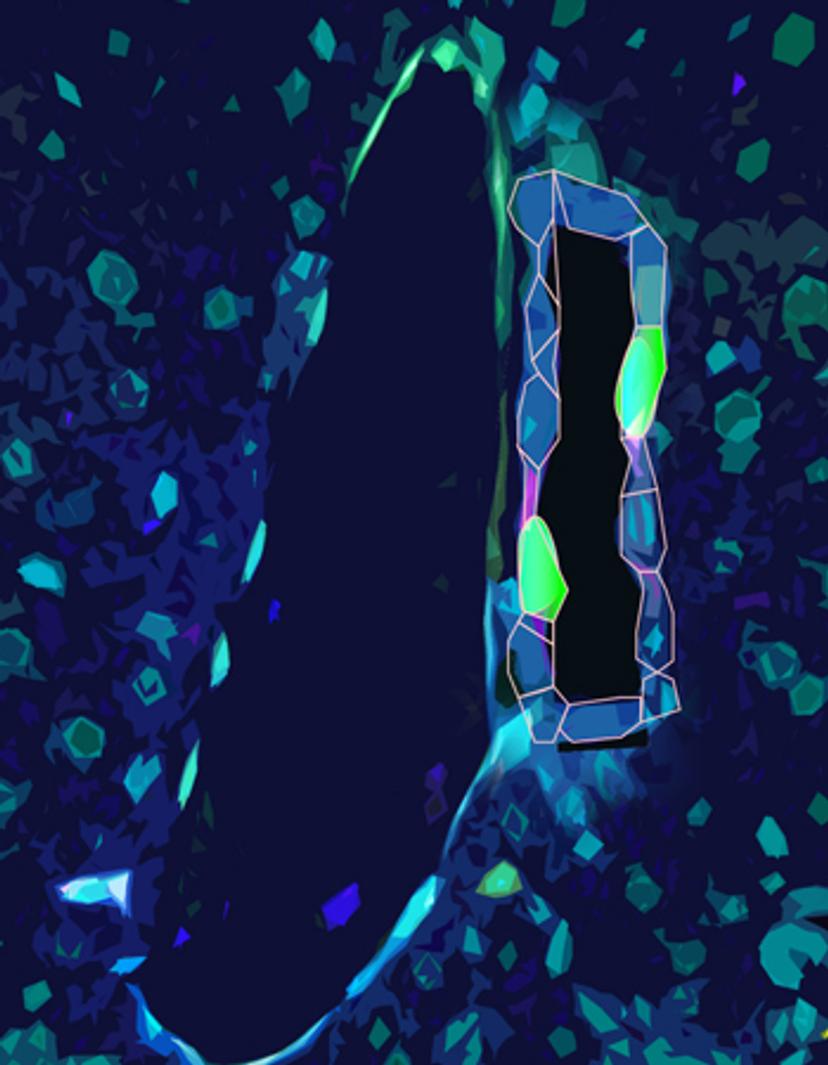
P-M: Previous work from Dr. Carmago’s laboratory has shown that over-activation of a signaling pathway, the Hippo-YAP signaling pathway, causes a reprogramming event in which hepatocytes became more like biliary epithelial cells, and possess more progenitor cell type characteristics. This suggests that hepatocytes within the liver have significant plasticity, that can be molecularly ‘switched on’, and more recent work builds on this by stitching together the significance of this signaling axis during regrowth and regeneration.
In our latest publication: ‘Single cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration’ we wanted to examine the pathways that regulate the liver epithelium in greater depth - specifically hepatocytes and biliary epithelial cells. We posited that understanding the molecular changes under both physiological conditions and during regeneration, at single-cell resolution, would help us to reveal the stem cell populations in the liver that serve as the source for its amazing regenerative capacity.
This study found that the activation of the protein YAP, even under physiologically normal conditions, is essential for the survival of the biliary cells. Additionally, we found that YAP is essential in hepatocytes in regulating the normal ductular response to liver injury. In total, our findings suggest that both types of liver cells are highly plastic; they have the unique ability to dynamically alter their signaling state to respond to injury.
SS: How did you manage to examine gene and protein expression patterns within a complex 3D organ, at the single-cell level?
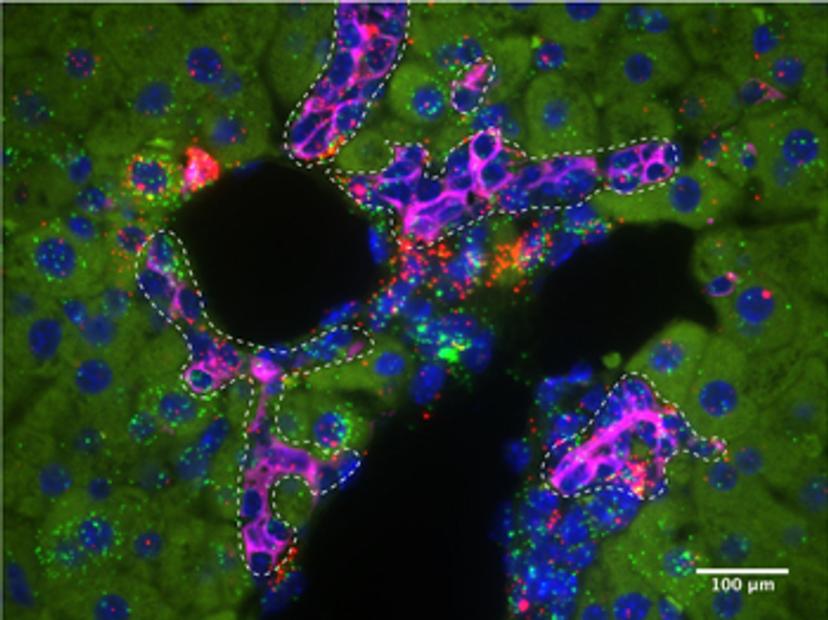
P-M: To carry out this type of work we used a myriad of murine genetic models, as well as histological analyses, including single-molecule fluorescence in situ hybridization, and single-cell omics. With the emergence of new single-cell sequencing techniques, we are beginning to understand the different signaling pathways which regulate regenerative cell states in exquisite detail. In our work, we took advantage of the RNAscope technology from Advanced Cell Diagnostics to understand signaling in different epithelial cell populations in the liver, during homeostasis and regeneration.
This technology enabled us to validate our single-cell RNA sequencing results. Previously we would have had to design several different RNA in situ probes for this validation. Using this technology, we were not only able to reproducibly examine the expression patterns of several different genes on our tissue sections, but we were also able to evaluate the co-localization of the expression of these genes using ACD’s dual-labeling technology.
SS: How could these findings improve the treatment of liver diseases, such as liver cancer, in the future?
P-M: We are at an incredibly exciting time for stem cell and regenerative biological research, and these findings, in particular, have exciting implications for cellular reprogramming during disease. The ability to modulate the signaling state of liver epithelial cells will potentially allow for future liver treatments in which scientists can program cells to more effectively respond to injury or replace diseased tissue.
Want more of the latest science news straight to your inbox? Become a member of SelectScience for free today>>

