News & Articles
Selected Filters:
Horizon Discovery expands cell-based CRISPR screening services
Horizon Discovery has announced a new B cell screening service to support the development of therapeutics for immune system diseases
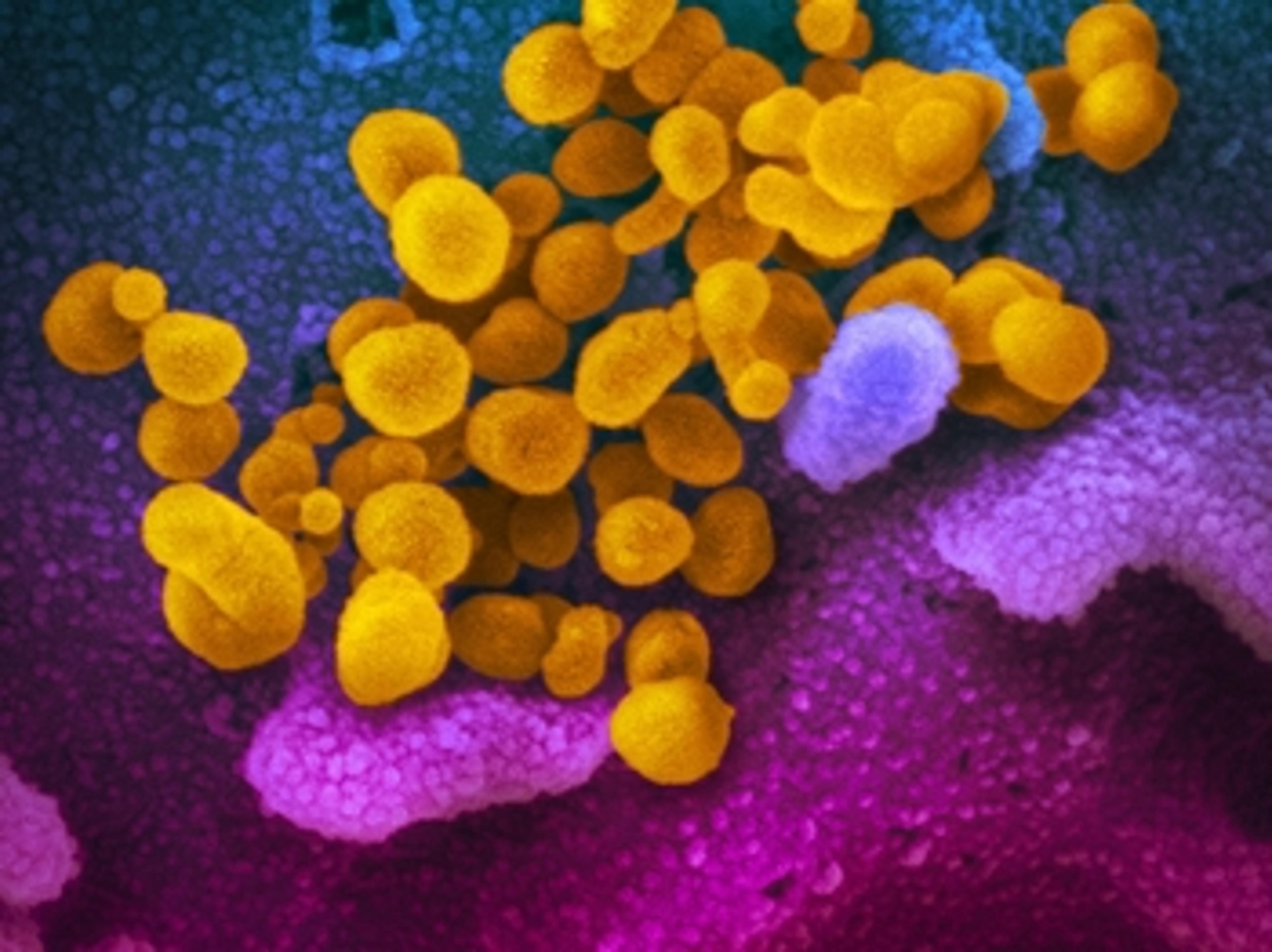
Bio-Rad receives FDA emergency use authorization for droplet digital PCR SARS-CoV-2 test kit
Test runs on Bio-Rad’s QX200 and QXDx ddPCR systems and aims to provide early detection of COVID-19
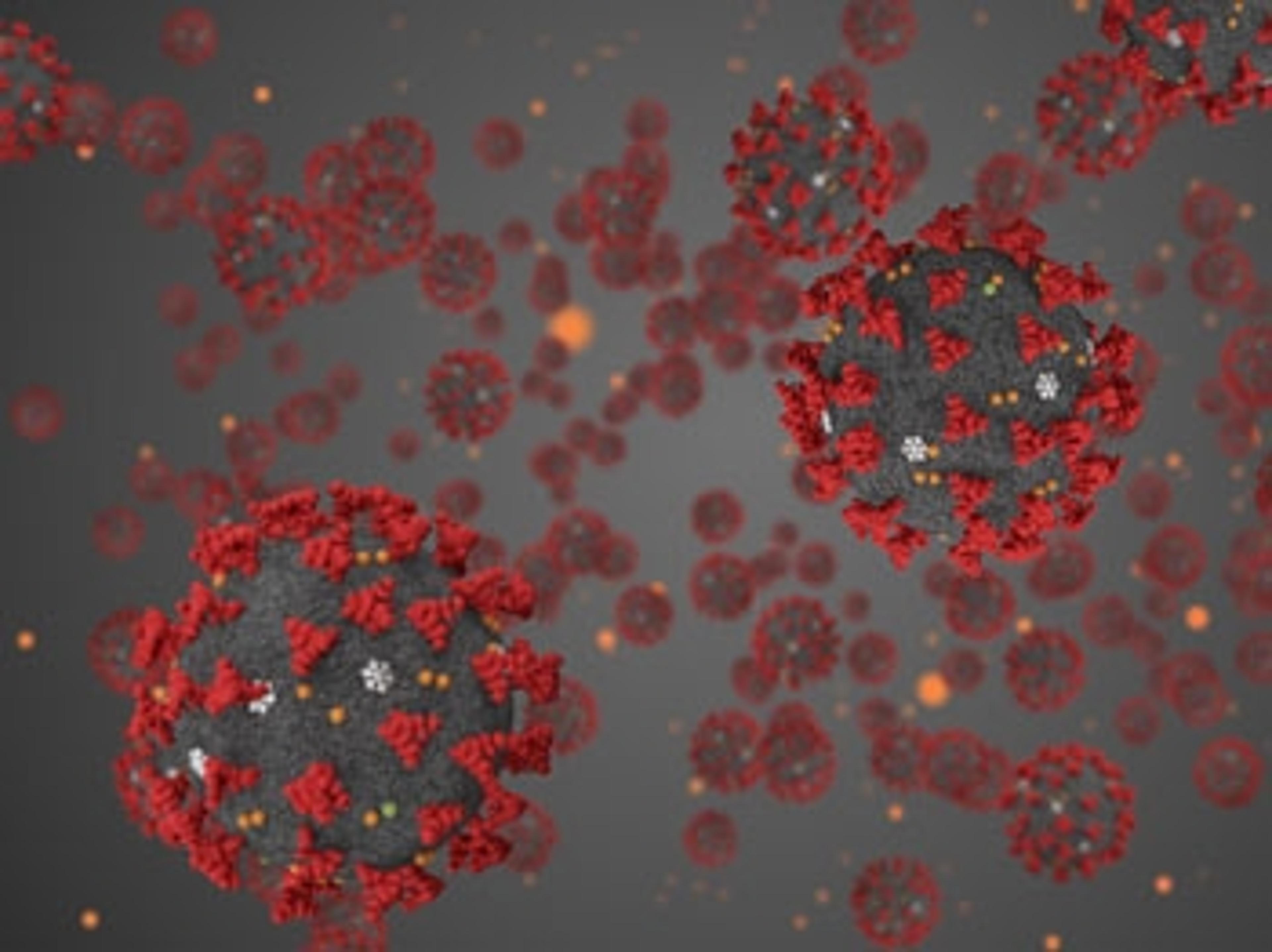
Bio-Rad's ddPCR shows greater sensitivity in COVID-19 detection studies
Studies in China show that the QX200 ddPCR systems can detect low levels of infection in individuals
PCR Biosystems increases operations for COVID-19 testing
PCR Biosystems have continued to scale up their operations to ensure the ongoing availability of COVID-19 test reagents
Bosch develops rapid test for combating the coronavirus
The test results are designed to enable differential diagnosis in under 2.5 hours
Luminex receives FDA emergency use authorization for COVID-19 diagnostic panel
Recent funding from the BARDA award helped the company accelerate development and validation
Rapid COVID-19 diagnostic test developed by Cambridge team to be deployed in hospitals
The SAMBA II machines provide a simple and accurate system for the diagnosis of COVID-19 infection
Oxford firms join forces to scale up production of COVID-19 antigens
In the wake of the pandemic, the strategic partnership aims to increase antigen production for diagnostic kits and vaccine development
Positive early-stage results for novel micro-RNA therapeutic for ischemic heart diseases
Horizon Discovery and Pharmahungary have reported a micro-RNA compound suitable for further development
FLIR launches smart thermal cameras to mitigate the spread of coronavirus
Initial shipments of FLIR A400/A700 thermal sensor solution will be prioritized for those responding to the COVID-19 virus
Beckman Coulter announces development of tests to detect coronavirus antibodies
New IgM and IgG antibody assays will help indicate whether a person has developed immunity against COVID-19
Clinical trials under way testing treatment options for COVID-19
The scientific community is working at full speed to fight the severe acute respiratory syndrome
PHCbi announces expedited delivery of laboratory products for COVID-19
The rapid delivery program is designed to provide critical laboratory equipment in this time of need
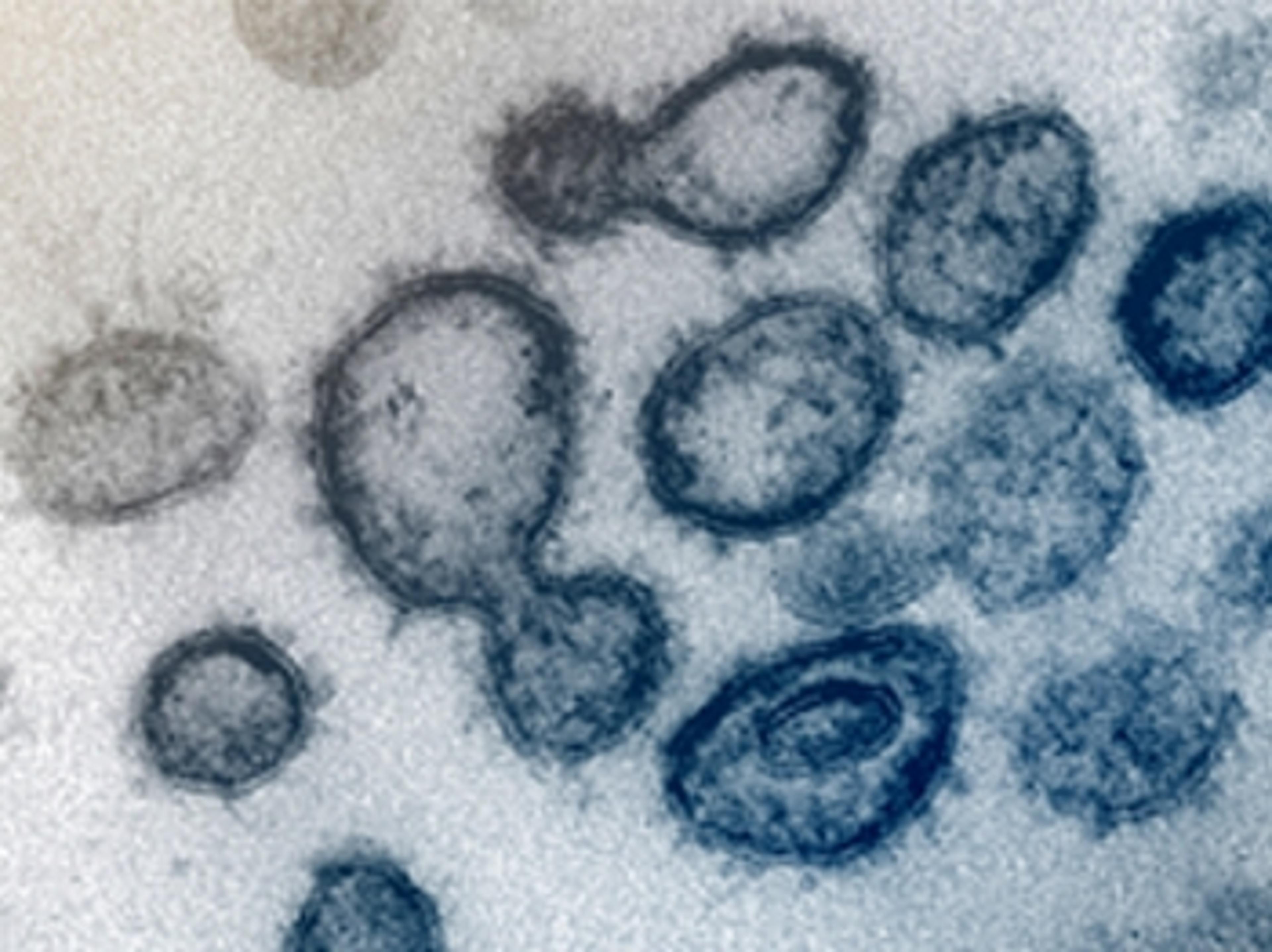
Learning from the recovered coronavirus patients
Samples from those who had COVID-19 could reveal true infection rate, lethality and vaccines
Bruker launches CE-IVD marked genesig assay kit for COVID-19 detection
The partnership with Primer Design aims to distribute the Genesig CE-IVD & FDA-EUA COVID-19 qPCR kit to Europe and the U.S.
Siemens Healthineers receives FDA clearance to support COVID-19 response efforts
The RAPIDPoint 500e blood gas analyzer aims to meet the unprecedented demand for blood gas testing in coronavirus patients
Cobra Biologics and the Karolinska Institutet collaborate to develop COVID-19 vaccine
OPENCORONA consortium awarded €3 million funding by Horizon 2020 to support emergency research and development against the COVID-19 outbreak
Abbott launches test that can detect COVID-19 in five minutes
The Abbott ID NOW™ COVID-19 test aims to bring rapid testing to the front lines of the global pandemic
EUROIMMUN to provide CE-marked antibody detection systems to support COVID-19 diagnostics
These are some of the first antibody detection tests to be CE marked in Europe and available for COVID-19 testing
First patients enrolled in new clinical trial of COVID-19 treatments
This clinical trial aims to test the effects of potential drug treatments for patients with the novel coronavirus
Cepheid receives FDA emergency use authorization for SARS-CoV-2 test
The Xpert Xpress SARS-CoV-2 test is designed for the qualitative detection of the virus causing COVID-19
AstraZeneca collaborates with Silence Therapeutics to develop siRNA therapeutics
The collaboration aims to develop novel, targeted treatments to address significant unmet needs
Coronavirus detection with Iceni Diagnostics technology offers potential for home test
Coronavirus is said to be detected using Iceni Diagnostics’ rapid, point-of-care screening technology, which distinguishes it from influenza